filmov
tv
Atomic Radius - Basic Introduction - Periodic Table Trends, Chemistry

Показать описание
This chemistry video tutorial provides a basic introduction into atomic radius which is one of the four main periodic table trends you need to know. Atomic radius increases as you down a group and to the left across the periodic table. Atomic radius decreases with effective nuclear charge but increases with each successive energy level added to an atom as you go down a group.
Ionization Energy:
Electron Affinity:
Atomic Radius:
Bond Energy & Bond Length:
Electronegativity:
Periodic Trends:
__________________________________
Polar & Nonpolar Covalent Bonding:
Bond Polarity & Dipole Moment:
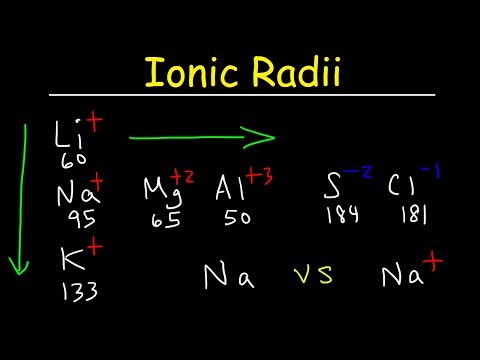
Ionic Radius:
Lattice Energy:
Born Haber Cycle:
Bond Energy Calculations:
___________________________________
Lewis Structures - Mega Review:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Ionization Energy:
Electron Affinity:
Atomic Radius:
Bond Energy & Bond Length:
Electronegativity:
Periodic Trends:
__________________________________
Polar & Nonpolar Covalent Bonding:
Bond Polarity & Dipole Moment:
Ionic Radius:
Lattice Energy:
Born Haber Cycle:
Bond Energy Calculations:
___________________________________
Lewis Structures - Mega Review:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:14:04
0:14:04
 0:07:53
0:07:53
 0:08:04
0:08:04
 0:11:47
0:11:47
 0:18:06
0:18:06
 0:03:47
0:03:47
 0:09:40
0:09:40
 0:05:04
0:05:04
 0:07:27
0:07:27
 0:05:22
0:05:22
 0:01:38
0:01:38
 0:17:39
0:17:39
 0:24:55
0:24:55
 0:25:57
0:25:57
 0:00:38
0:00:38
 0:00:54
0:00:54
 0:09:49
0:09:49
 0:03:28
0:03:28
 0:01:31
0:01:31
 0:24:37
0:24:37
 0:00:48
0:00:48
 0:00:54
0:00:54
 0:10:18
0:10:18
 0:07:40
0:07:40