filmov
tv
Derivation Kinetic Energy = (3/2)nRT

Показать описание
The KE=(1/2)mv2 video link is
This video is a very quick, all math, derivation of K.E.=(3/2)nRT=(3/2)PV.
This is primarily a memory refresher. If it is your first exposure to this material, then it may be a little too quick.
This video is a very quick, all math, derivation of K.E.=(3/2)nRT=(3/2)PV.
This is primarily a memory refresher. If it is your first exposure to this material, then it may be a little too quick.
Derivation Kinetic Energy = (3/2)nRT
Prove that the kinetic energy per molecule of an ideal gas is 3/2kbt | simplified
Molecular Kinetic Theory (simple derivation) - Kinetic Theory (Lesson 4)
Derivation of relationship between Kinetic energy per mole of a gas and Temperature.
Derivation of 3:2kT
Kinetic Molecular Theory and the Ideal Gas Laws
Kinetic energy= 3/2 nRT #shorts #short #shortvideo #shortsvideo #viral #shortsfeed @GyanFreedom
Mean Kinetic Energy of Gas Molecules - Kinetic Theory (Lesson 5)
Internal Energy of an Ideal Gas - Thermal Physics (Lesson 6)
11.3.5 Derivation of Internal Energy Formula for Ideal Monatomic Gas
U=3/2 nRT PROOF | KE = 3/2nRT | KE Equals 3 divide by 2 nRT for a monoatomic ideal gas. - Kisembo
How To Calculate The Average Translational Kinetic Energy of Molecules Using Boltzmann's Consta...
Feeling the Pressure of the Ideal Gas Law
How to derive the ideal gas equation -Equation for ideal gas (PV=nRT) - PV =nRT derivation - Kisembo
PV=nRT derivation - How to derive the ideal gas equation from P=1/3(Mn/V)C^2 (Kinetic Pressure)
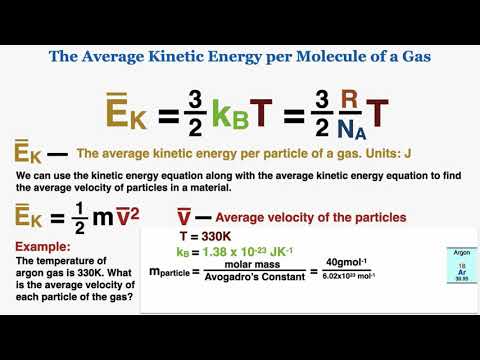
The Average Kinetic Energy per Molecule Equation for an Ideal Gas - IB Physics
Translational Kinetic Energy | Derivation Of Translational Kinetic energy
Deriving Gas Pressure Equation - Ideal Gas Laws - temperature is proportional to kinetic energy
Gas Law Formulas and Equations - College Chemistry Study Guide
Boyle’s Law
UPSC VS IIT JEE 🥵 #iitstatus #motivation #toppers #iitjee #jeemains #upscstatus #neet #nit #jee
In the formula `p = 2/3 E`, the term (E) represents translational kinetic energy per unit volume...
Full Derivation of PV=nRT
Derivation of PV=nRT
Комментарии
 0:06:16
0:06:16
 0:03:28
0:03:28
 0:04:13
0:04:13
 0:02:45
0:02:45
 0:09:52
0:09:52
 0:05:11
0:05:11
 0:00:29
0:00:29
 0:03:17
0:03:17
 0:02:15
0:02:15
 0:04:10
0:04:10
 0:19:31
0:19:31
 0:06:47
0:06:47
 0:00:18
0:00:18
 0:04:27
0:04:27
 0:06:34
0:06:34
 0:05:38
0:05:38
 0:02:06
0:02:06
 0:10:13
0:10:13
 0:19:24
0:19:24
 0:00:15
0:00:15
 0:00:14
0:00:14
 0:04:18
0:04:18
 0:09:53
0:09:53
 0:13:16
0:13:16