filmov
tv
Prove that the kinetic energy per molecule of an ideal gas is 3/2kbt | simplified

Показать описание
prove that the kinetic energy per molecule of an ideal gas is 3/2kbt
kinetic theory of gases and radiation
#physics
#class12
#kinetic
#bholanathacademy
#prove
#proof
#kineticenergy
#radiation
#3
#kbt
#clear
#exam
#physicssimplified
derive kinetic energy per molecule of an ideal gas is 3/2 kbt
kinetic energy per molecule of an ideal gas is 3/2 kbt
prove that the kinetic energy per molecule of an ideal gas is 3/2kbt
How do you prove that the average kinetic energy of a molecule is proportional to the absolute temperature of the gas?
How do you prove average kinetic energy of molecule is directly proportional to absolute temperature 10th class?
How is the average kinetic energy of a molecule of an ideal gas?
Which statement describes the particles of an ideal gas based on the kinetic molecular theory?
How that the average energy of the molecules of gas is directly proportional to the absolute temperature of gas?
What are the 3 postulates of kinetic theory of gases?
kinetic theory of gases and radiation
#physics
#class12
#kinetic
#bholanathacademy
#prove
#proof
#kineticenergy
#radiation
#3
#kbt
#clear
#exam
#physicssimplified
derive kinetic energy per molecule of an ideal gas is 3/2 kbt
kinetic energy per molecule of an ideal gas is 3/2 kbt
prove that the kinetic energy per molecule of an ideal gas is 3/2kbt
How do you prove that the average kinetic energy of a molecule is proportional to the absolute temperature of the gas?
How do you prove average kinetic energy of molecule is directly proportional to absolute temperature 10th class?
How is the average kinetic energy of a molecule of an ideal gas?
Which statement describes the particles of an ideal gas based on the kinetic molecular theory?
How that the average energy of the molecules of gas is directly proportional to the absolute temperature of gas?
What are the 3 postulates of kinetic theory of gases?
Kinetic energy derivation
Great science teacher risks his life explaining potential and kinetic energy
How to Derive Kinetic Energy Quickly - A Level Physics
kinetic energy equation proof and explanation
Can you derive the formula for Kinetic Energy?
Derivation of kinetic energy, learn physics with fun.
Why is there a half in kinetic energy formula ? Work- energy principle.
Easy way to prove that work done is change in kinetic energy
Shocking Link Between QUANTUM MECHANICS and CONSCIOUSNESS Unveiled by a Nuclear Physicist!
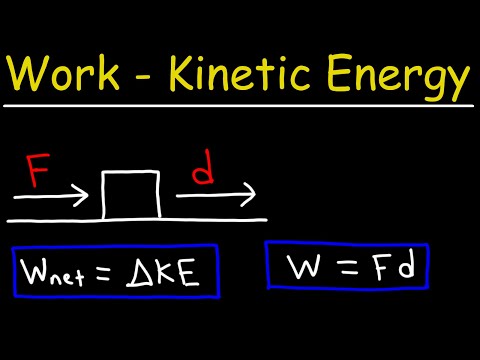
Work and Kinetic Energy - Physics
Deriving the Work-Energy Theorem using Calculus
Class 9 - Physics Chapter 6 - Lecture 2 - Energy & Kinetic Energy - Allied Schools
Prove that the kinetic energy per molecule of an ideal gas is 3/2kbt | simplified
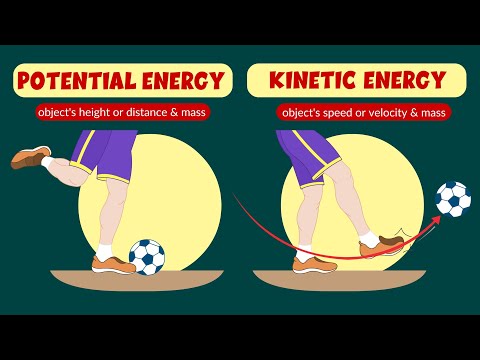
Potential and Kinetic Energy | #aumsum #kids #science #education #children
Potential and kinetic energy - Law of conservation of energy - Video for kids
Prove that the kinetic energy operator is a Hermitian Operator
Kinetic Energy Pythagorean Theorem Proof! (Math w/ Physics #2)
Derivation of mathematical expression of Kinetic Energy, KE= 1/2 mv^2
Math Proofs | Kinetic Energy Formula Proof Using Calculus
Kinetic Energy | Mathematical proof of kinetic energy |unit 6 | work and energy
Derivation Kinetic Energy = (3/2)nRT
Prove that the kinetic energy of a freely falling object on reaching the ground is nothing but the
Derivation of Kinetic Energy | Class 9 | Work Power and Energy,
Einstein's Proof of E=mc²
Комментарии
 0:03:27
0:03:27
 0:03:19
0:03:19
 0:01:28
0:01:28
 0:06:22
0:06:22
 0:04:43
0:04:43
 0:04:10
0:04:10
 0:05:40
0:05:40
 0:04:27
0:04:27
 1:05:19
1:05:19
 0:13:05
0:13:05
 0:07:54
0:07:54
 0:15:47
0:15:47
 0:03:28
0:03:28
 0:04:28
0:04:28
 0:03:56
0:03:56
 0:13:01
0:13:01
 0:03:44
0:03:44
 0:03:23
0:03:23
 0:12:08
0:12:08
 0:13:12
0:13:12
 0:06:16
0:06:16
 0:00:39
0:00:39
 0:03:49
0:03:49
 0:02:11
0:02:11