filmov
tv
Molecular Kinetic Theory (simple derivation) - Kinetic Theory (Lesson 4)
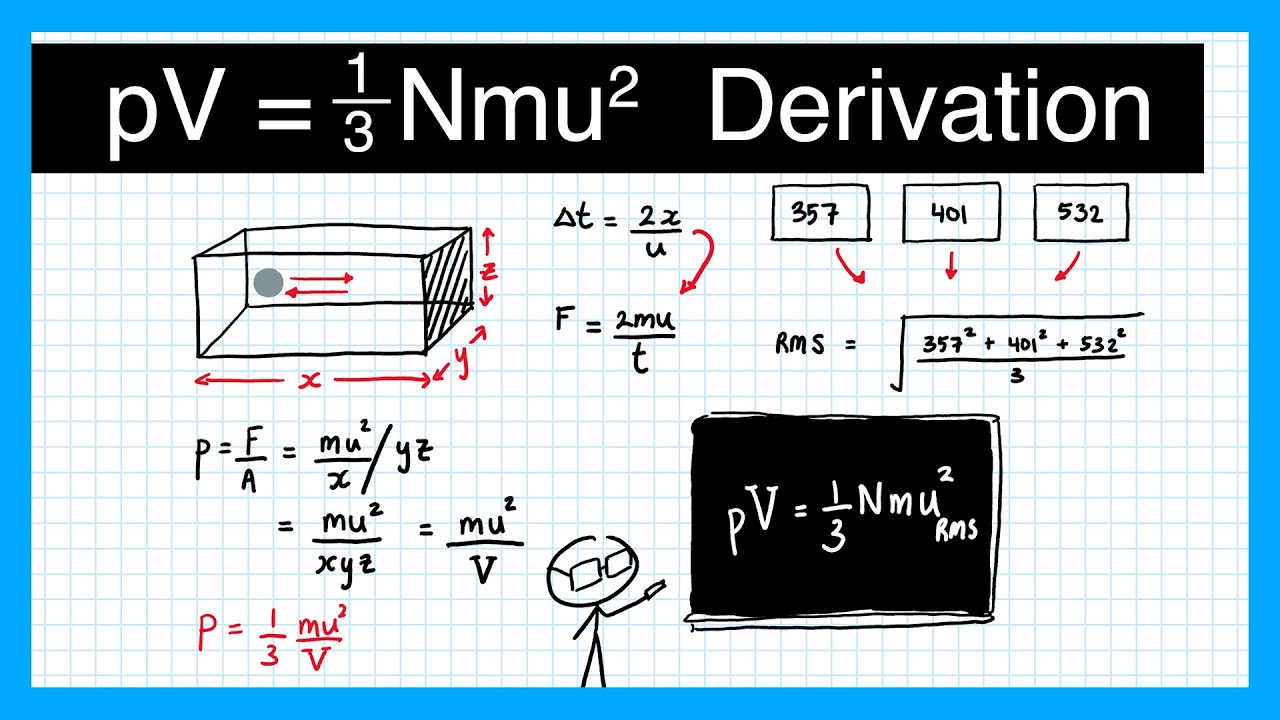
Показать описание
Lesson 4
The kinetic theory of gas allows us to derive the equation of gas pressure pV=1/3 Nmu^2.
In this video, we look at what the root mean square speed is and how that is used in the gas pressure equation.
Lesson 3
Two versions of the empirical ideal gas equation:
pV = nRT
pV = NkT
Lesson 2
Boyle's law, Charles' law, and Gay-Lussac's law, and the combined gas law
Lesson 1
Describing an Ideal Gas - Moles, Molar Mass, Relative Molar Mass, Avogadro Constant
#physics #kinetictheory #alevelphysics #neetphysics #class11
Music credit:
Song: Dipcrusher - Islands (Vlog No Copyright Music)
Music provided by Vlog No Copyright Music.
The kinetic theory of gas allows us to derive the equation of gas pressure pV=1/3 Nmu^2.
In this video, we look at what the root mean square speed is and how that is used in the gas pressure equation.
Lesson 3
Two versions of the empirical ideal gas equation:
pV = nRT
pV = NkT
Lesson 2
Boyle's law, Charles' law, and Gay-Lussac's law, and the combined gas law
Lesson 1
Describing an Ideal Gas - Moles, Molar Mass, Relative Molar Mass, Avogadro Constant
#physics #kinetictheory #alevelphysics #neetphysics #class11
Music credit:
Song: Dipcrusher - Islands (Vlog No Copyright Music)
Music provided by Vlog No Copyright Music.
Molecular Kinetic Theory (simple derivation) - Kinetic Theory (Lesson 4)
Kinetic molecular theory of gases | Physical Processes | MCAT | Khan Academy
Kinetic Theory of Gases - A-level Physics
Kinetic Molecular Theory and the Ideal Gas Laws
Kinetic Molecular Theory of Gases
Kinetic Molecular Theory and its Postulates
Kinetic Theory Derivations: Thermodynamics: Edexcel A-level Physics
The Kinetic Molecular Theory (Animation)
Derivation of kinetic theory of gases| complete derivation with easy understanding|
Derivation of kinetic theory AQA Alevel Physics
Kinetic Molecular Theory of Gases - Practice Problems
Kinetic Molecular Theory & Ideal Gas Law Derivation
Prove that the kinetic energy per molecule of an ideal gas is 3/2kbt | simplified
Boyle’s Law
Molecular Kinetic Theory
Pressure of an Ideal Gas | KTG Derivation | YOLO JEE Advance Physics with Vikrant Kirar
Derive P = 1/3 mnv^-2 using the kinetic theory of gas
Relationship between pressure and temperature (derivation, kinetic theory of gases)
Pressure of a Gas on the basis of Kinetic Theory | A Molecular View of Pressure
Average Kinetic Energy of a Gas and Root Mean Square Velocity Practice Problems - Chemistry Gas Laws
Derivation of relationship between Kinetic energy per mole of a gas and Temperature.
Kinetic theory of Gases Pressure 3D animation of the Derivation only Visual no math part
The kinetic molecular theory of gases | AP Chemistry | Khan Academy
Kinetic Theory of Matter | Chemistry
Комментарии
 0:04:13
0:04:13
 0:14:56
0:14:56
 0:11:28
0:11:28
 0:05:11
0:05:11
 0:08:10
0:08:10
 0:07:00
0:07:00
 0:09:54
0:09:54
 0:01:31
0:01:31
 0:13:59
0:13:59
 0:14:12
0:14:12
 0:43:21
0:43:21
 0:16:49
0:16:49
 0:03:28
0:03:28
 0:00:15
0:00:15
 0:24:45
0:24:45
 0:25:24
0:25:24
 0:00:11
0:00:11
 0:17:58
0:17:58
 0:12:42
0:12:42
 0:12:51
0:12:51
 0:02:45
0:02:45
 0:04:03
0:04:03
 0:06:24
0:06:24
 0:03:38
0:03:38