filmov
tv
Orbital - Chem Definition

Показать описание
Orbital - Chem Definition
Quantum Numbers, Atomic Orbitals, and Electron Configurations
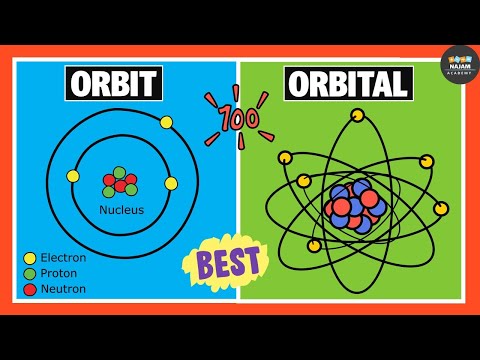
Difference Between Orbits and Orbitals | Chemistry
What are Shells, Subshells, and Orbitals? | Chemistry
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
Orbital Definition #chemistry #science #shorts #youtubeshorts
Atomic orbitals 3D
Atomic Orbitals
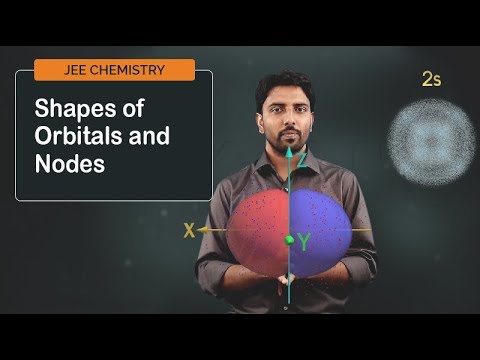
JEE 2023 Chemistry Concepts Explained | Shapes of Orbitals and Nodes
How small are atoms?
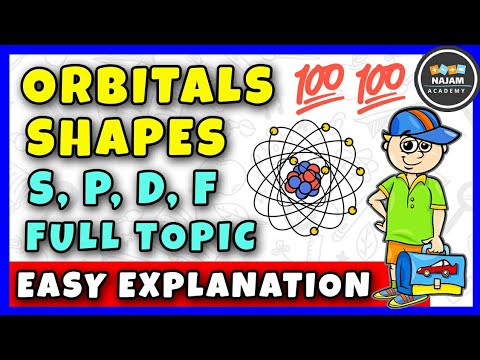
The Shapes of Atomic Orbitals s-orbital, p-orbital and d-orbital
Concept of orbital || Difference between orbit and orbital || 11th class chemistry || ch.no.5
Quantum Numbers
Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy
What is an Orbital? 🤔
Definition of Orbital
Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle - Electron Configur...
What are orbits and orbitals??🤔🤔#differences between orbit and orbital#chemistry.
Define orbital.
Electron Configuration - Basic introduction
orbit/shell, subshell & Orbital
s-orbital || Shapes of orbitals || 11th class chemistry || Ch.no.5
Hybridization of Atomic Orbitals | SP, SP2, SP3 Hybridization of Carbon
What is | Difference between Shell, Sub shell and Orbital | Structure of Atom Kya Hai | Chemistry
Комментарии
 0:08:42
0:08:42
 0:08:11
0:08:11
 0:06:00
0:06:00
 0:11:19
0:11:19
 0:00:32
0:00:32
 0:05:50
0:05:50
 0:02:50
0:02:50
 0:02:50
0:02:50
 0:00:48
0:00:48
 0:10:53
0:10:53
 0:20:44
0:20:44
 0:12:16
0:12:16
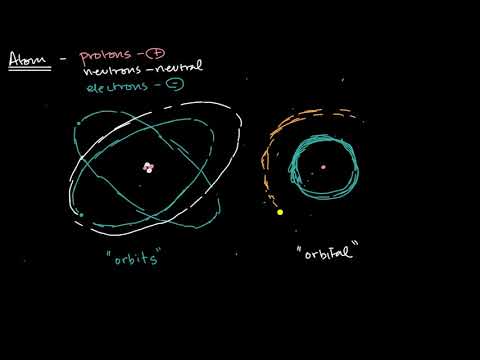 0:09:41
0:09:41
 0:00:41
0:00:41
 0:01:27
0:01:27
 0:05:24
0:05:24
 0:00:56
0:00:56
 0:01:26
0:01:26
 0:10:19
0:10:19
 0:00:11
0:00:11
 0:06:05
0:06:05
 0:13:48
0:13:48
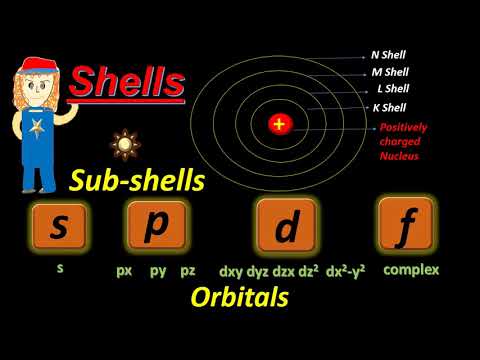 0:06:04
0:06:04