filmov
tv
Hybridization in Chemistry - Full Lecture with Examples

Показать описание
Full hybridization in chemistry lecture with example at the end of the lecture of identifying hybridization in organic molecules and inorganic molecules. Sp3, sp2, sp hybridization are explained with visual examples including how hybridizations lead to geometries. Sigma and pi bonding is also explained.
Video Thumbnail: Atomic orbitals mixing, much like a Venn diagram or the circles in the photo.
Video Thumbnail Photo Credit: MS365 Powerpoint
#chemistry
#hybridization
#hybridorbitals
See time stamps below.
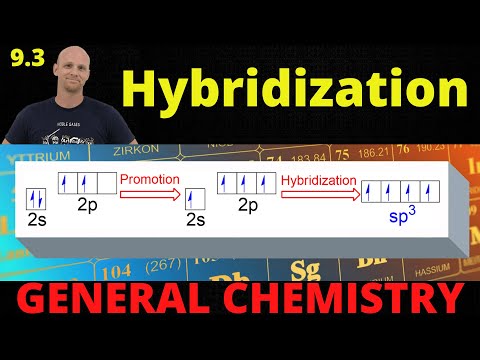
02:19 sp3 hybridization explained
04:08 sp3 hybridization has tetrahedral geometry
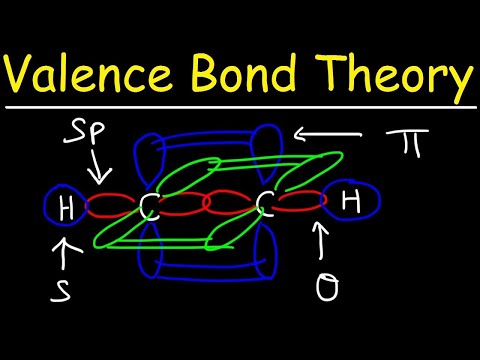
04:35 sigma bonds explained
05:00 sp2 hybridization explained
06:33 sp2 hybridization has trigonal planar geometry
08:07 pi bonds explained
10:10 sp hybridization explained
10:38 sp hybridization has linear geometry
12:06 sigma and pi bonds between sp hybridized atoms
13:01 d orbital hybridization
13:16 dsp3 hybridization explained
13:32 d2sp3 hybridization explained
15:19 rules for predicting hybridization
16:05 table of hybridization and electron pair geometries
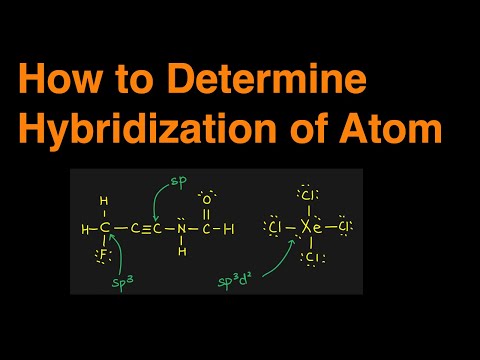
16:46 determining hybridizations example 1
21:27 determining hybridizations example 2
23:49 determining hybridizations example 3
25:43 determining hybridizations example 4 PCl5
26:57 determining hybridizations example 5 XeF4
Images: Microsoft powerpoint and wiki commons
Video Thumbnail: Atomic orbitals mixing, much like a Venn diagram or the circles in the photo.
Video Thumbnail Photo Credit: MS365 Powerpoint
#chemistry
#hybridization
#hybridorbitals
See time stamps below.
02:19 sp3 hybridization explained
04:08 sp3 hybridization has tetrahedral geometry
04:35 sigma bonds explained
05:00 sp2 hybridization explained
06:33 sp2 hybridization has trigonal planar geometry
08:07 pi bonds explained
10:10 sp hybridization explained
10:38 sp hybridization has linear geometry
12:06 sigma and pi bonds between sp hybridized atoms
13:01 d orbital hybridization
13:16 dsp3 hybridization explained
13:32 d2sp3 hybridization explained
15:19 rules for predicting hybridization
16:05 table of hybridization and electron pair geometries
16:46 determining hybridizations example 1
21:27 determining hybridizations example 2
23:49 determining hybridizations example 3
25:43 determining hybridizations example 4 PCl5
26:57 determining hybridizations example 5 XeF4
Images: Microsoft powerpoint and wiki commons
 0:10:55
0:10:55
 0:13:48
0:13:48
 0:16:52
0:16:52
 0:07:54
0:07:54
 1:29:10
1:29:10
 0:04:08
0:04:08
 0:08:22
0:08:22
 1:38:48
1:38:48
 0:04:29
0:04:29
 0:10:39
0:10:39
 0:11:58
0:11:58
 0:07:43
0:07:43
 0:56:46
0:56:46
 0:00:45
0:00:45
 0:10:52
0:10:52
 0:44:07
0:44:07
 1:00:18
1:00:18
 0:38:29
0:38:29
 0:01:00
0:01:00
 0:06:17
0:06:17
 0:03:35
0:03:35
 0:31:49
0:31:49
 0:15:55
0:15:55
 0:16:23
0:16:23