filmov
tv
sp3 hybridized orbitals and sigma bonds | Structure and bonding | Organic chemistry | Khan Academy

Показать описание
sp3 Hybridized Orbitals and Sigma Bonds. Created by Sal Khan.
Organic Chemistry on Khan Academy: Carbon can form covalent bonds with itself and other elements to create a mind-boggling array of structures. In organic chemistry, we will learn about the reactions chemists use to synthesize crazy carbon based structures, as well as the analytical methods to characterize them. We will also think about how those reactions are occurring on a molecular level with reaction mechanisms. Simply put, organic chemistry is like building with molecular Legos. Let's make some beautiful organic molecules!
For free. For everyone. Forever. #YouCanLearnAnything
sp3 hybridized orbitals and sigma bonds | Structure and bonding | Organic chemistry | Khan Academy
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
sp3, sp2, sp hybridization for DUMMIES
Hybridization of Atomic Orbitals | SP, SP2, SP3 Hybridization of Carbon
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
sp3 Hybridized Orbitals and Sigma Bonds
Quick Hybridization Review #organicchemistry #chemistry
Class 11th -Chemistry- 'Chemical Bonding' - 'ONE SHOT' NCERT & Exempler Cove...
Orbitals: Crash Course Chemistry #25
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry
Orbitalemodell - Sp3 - Hybridisation
Organic chemistry - sp3 hybridized orbitals and sigma bonds
Orbital Hybridization sp3 sp2 sp sigma
013 sp3 Hybridized Orbitals and Sigma Bonds
How to determine Hybridization - s, sp, sp2, and sp3 - Organic Chemistry
sp3 Hybrid Orbitals
sp3 hybridized orbitals in methane, diamond
Sp,Sp2 Hybridization
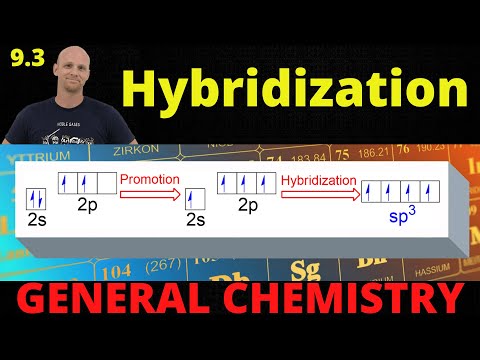
9.3 Hybridization | General Chemistry
sp3 hybridized orbitals and sigma bonds | Structure and bonding | Organic chemistry | KA Urdu
| SP3 Hybridization | | My Inter Academy |
S2.2.15 Explain hybridization as mixing of orbitals making new orbitals [HL IB Chemistry]
sp2 and sp3 Hybridisation of Carbon (A-level and IB Chemistry)
Комментарии
 0:16:23
0:16:23
 0:10:55
0:10:55
 0:00:45
0:00:45
 0:13:48
0:13:48
 0:11:58
0:11:58
 0:07:54
0:07:54
 0:16:23
0:16:23
 0:01:00
0:01:00
 2:14:18
2:14:18
 0:10:52
0:10:52
 0:06:17
0:06:17
 0:04:16
0:04:16
 0:16:23
0:16:23
 0:19:19
0:19:19
 0:16:23
0:16:23
 0:08:22
0:08:22
 0:13:45
0:13:45
 0:08:24
0:08:24
 0:01:08
0:01:08
 0:16:52
0:16:52
 0:16:11
0:16:11
 0:03:08
0:03:08
 0:05:28
0:05:28
 0:10:57
0:10:57