filmov
tv
Geometric Stereoisomerism: Cis/Trans and E/Z

Показать описание
This video outline how to identify cis/trans and E/Z geometric stereoisomers and includes detailed 3D molecules to help improve understanding. For geometric isomerism to exist both carbons of the C=C bond must have different groups attached. For example CH3CH=CHCH3 or ClCH=CH(OH). Where both carbons also have a H atom in common we can use the cis/trans method of identification. Otherwise it is necessary to use the E/Z method.
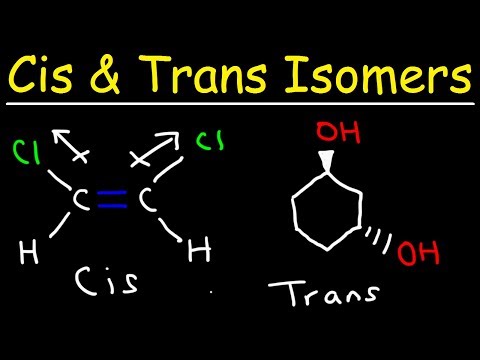
Cis and Trans Isomers
Geometric Stereoisomerism: Cis/Trans and E/Z
Using Cis/Trans versus E/Z to Describe Double Bonds
cis-trans and E-Z naming scheme for alkenes | Alkenes and Alkynes | Organic chemistry | Khan Academy
E/Z Absolute Configuration of Alkenes
E Z Geometric Isomers for Alkenes
Cis–trans isomerism | Alkenes and Alkynes | Organic chemistry | Khan Academy
Cis Trans Geometric Isomers for Alkenes and Cyclohexane
Geometric Isomers | Organic Chemistry | Cis/Trans | E/Z
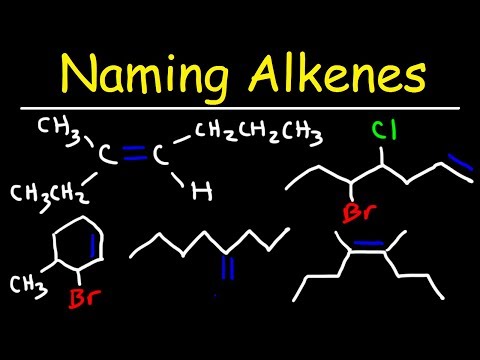
Naming Alkenes Using E Z System - IUPAC Nomenclature
Geometrical Isomers of Alkenes (Cis- & trans- and Z- & E-)
Stereoisomers
E and Z Isomerism and Cis/Trans Stereoisomerism in ALKENES - OCR A - A level Chemistry
AS Chemistry. Geometric isomerism cis-trans and E Z. Video 1 of 2.
Cis-Trans (Z/E) Isomers
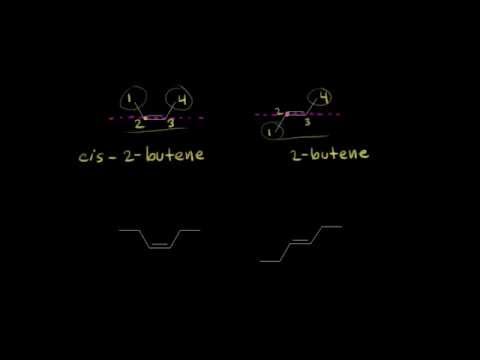
13a: Classifying alkenes as cis or trans
Determining cis/trans on cyclohexanes
E - Z system for naming geometric isomers
Cis-trans system for naming geometric isomers
Naming cis/trans Alkenes (E/Z too)
ChemDoodle Shorts: Geometric Isomers - E/Z
Stereoisomerism: Geometric Isomers & Optical Isomers
Stereoisomerism
cis- and trans- geometric isomerism
Комментарии
 0:06:35
0:06:35
 0:09:57
0:09:57
 0:15:21
0:15:21
 0:04:56
0:04:56
 0:06:01
0:06:01
 0:12:07
0:12:07
 0:05:24
0:05:24
 0:11:15
0:11:15
 0:10:48
0:10:48
 0:12:18
0:12:18
 0:21:58
0:21:58
 0:05:48
0:05:48
 0:04:13
0:04:13
 0:16:27
0:16:27
 0:05:30
0:05:30
 0:10:27
0:10:27
 0:09:14
0:09:14
 0:10:04
0:10:04
 0:05:06
0:05:06
 0:10:01
0:10:01
 0:05:12
0:05:12
 0:05:25
0:05:25
 0:13:34
0:13:34
 0:11:19
0:11:19