filmov
tv
Thermodynamics - Adiabatic Process | Adiabatic Expansion & Contraction | PV Diagram in Hindi / Urdu

Показать описание
Adiabatic Process Thermodynamics | Work done Adiabaticaly | Adiabatic Expansion & Contraction | PV Diagram in Hindi / Urdu
An adiabatic process is a thermodynamics process that occurs without the transfer of heat and mass between the system and sorrounding.
Thermodynamics is the branch of science which deals with the relationship between heat energy and other forms of energy.
Thermodynamics Playlist:
----------------------------------------------------------------------------------------------------------------------------------
An adiabatic process is a thermodynamics process that occurs without the transfer of heat and mass between the system and sorrounding.
Thermodynamics is the branch of science which deals with the relationship between heat energy and other forms of energy.
Thermodynamics Playlist:
----------------------------------------------------------------------------------------------------------------------------------
Adiabatic Process - Work, Heat & Internal Energy, Gamma Ratio, Thermodynamics & Physics
Adiabatic processes
Thermodynamic Processes (Animation)
Adiabatic Processes
Adiabatic Process with Ideal Gas - First Law of Thermodynamics Derivation (Integration, Natural Log)
Adiabatic Expansion: Cloud in the Bottle -- xmdemo 004
Adiabatic Process ( Classroom Demonstration)
Adiabatic Heating Demo
Adiabatic Process Explained in Hindi #neet #physicswallah #thermodynamics
The First Law of Thermodynamics: Internal Energy, Heat, and Work
Thermodynamic Processes: Isobaric, Isochoric, Isothermal and Adiabatic process | Chemistry #12
Isobaric, Isochoric, Isothermal, Adiabatic?
First Law of Thermodynamics - Adiabatic Process
PV diagrams - part 2: Isothermal, isometric, adiabatic processes | MCAT | Khan Academy
Adiabatic Process - What Is Adiabatic Process
Thermodynamics: Crash Course Physics #23
Thermodynamics - Adiabatic Process | Adiabatic Expansion & Contraction | PV Diagram in Hindi / U...
ADIABATIC EXPANSION - CLOUD IN THE BOTTLE
Thermodynamics and P-V Diagrams
Adiabatic Expansion/Compression
Reversible, Adiabatic Process - ISENTROPIC process in 3 Minutes!
The Adiabatic Process Explained
Adiabatic Cooling Animation
Class 11th - Thermodynamics Process - Adiabatic | Thermodynamics | Tutorials Point
Комментарии
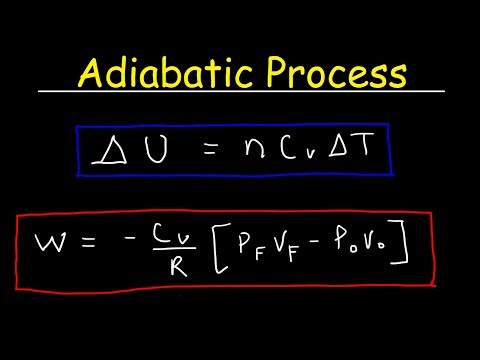 0:10:38
0:10:38
 0:03:58
0:03:58
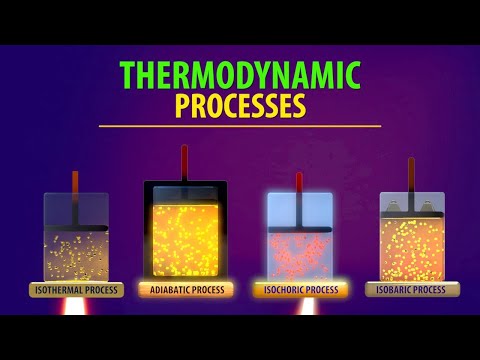 0:09:19
0:09:19
 0:10:46
0:10:46
 0:16:00
0:16:00
 0:00:38
0:00:38
 0:03:39
0:03:39
 0:02:05
0:02:05
 0:00:59
0:00:59
 0:05:44
0:05:44
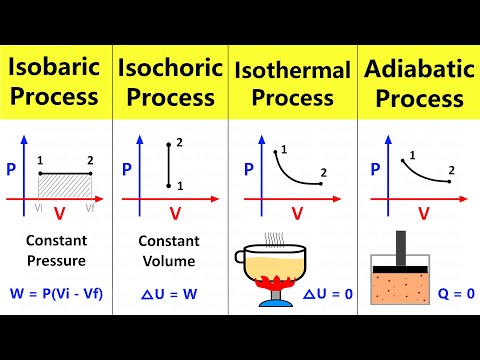 0:02:53
0:02:53
 0:02:52
0:02:52
 0:06:57
0:06:57
 0:13:00
0:13:00
 0:04:10
0:04:10
 0:10:04
0:10:04
 0:05:01
0:05:01
 0:00:36
0:00:36
 0:07:53
0:07:53
 0:01:46
0:01:46
 0:02:58
0:02:58
 0:00:48
0:00:48
 0:00:10
0:00:10
 0:13:39
0:13:39