filmov
tv
Thermodynamics and P-V Diagrams
Показать описание
085 - Thermodynamics and P-V Diagrams
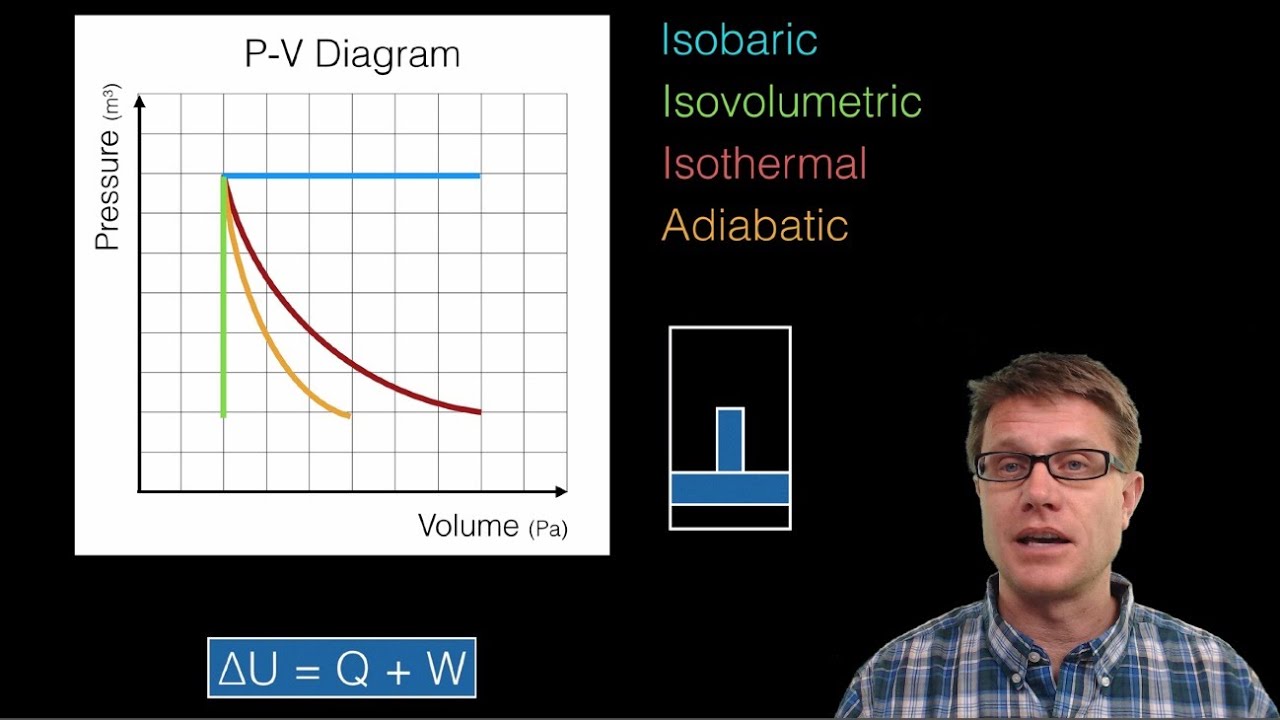
In this video Paul Andersen explains how the First Law of Thermodynamics applies to an ideal gas in a piston. A pressure-volume graph can be used to determine the type of thermodynamic process. Included is a discussion of and P-V diagram for isothermal, isovolumetric, isobaric, and adiabatic process.
In this video Paul Andersen explains how the First Law of Thermodynamics applies to an ideal gas in a piston. A pressure-volume graph can be used to determine the type of thermodynamic process. Included is a discussion of and P-V diagram for isothermal, isovolumetric, isobaric, and adiabatic process.
Thermodynamics and P-V Diagrams
PV Diagrams, How To Calculate The Work Done By a Gas, Thermodynamics & Physics
PV diagrams - part 1: Work and isobaric processes | Chemical Processes | MCAT | Khan Academy
T-v Diagrams and PROPERTY TABLES for Thermodynamics in 13 Minutes!
Pure Substances - P V Diagram
Thermodynamics- TV , PV diagrams, and properties example
Thermodynamic Processes: Isobaric, Isochoric, Isothermal and Adiabatic process | Chemistry #12
Thermodynamics - A-level Physics
Physics Adiabatic process 14 10 24 #Shorts #YouTube #Trending #Viral #News #Headlines
Thermodynamics L1 P-V diagram
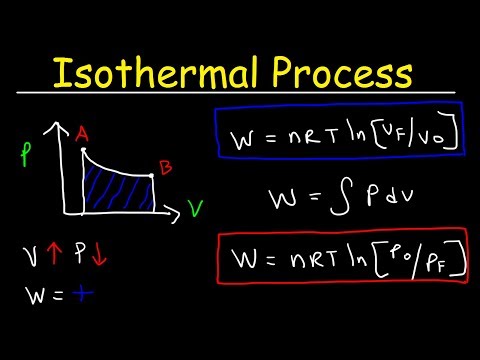
Isothermal process Thermodynamics - Work, Heat & Internal Energy, PV Diagrams
Thermodynamics: T-v Diagrams
Physics Review: Thermodynamics #22 PV-Diagram
Thermodynamics - Introduction to Phase, p-v and T-v Diagrams
Thermodynamics - PV Diagram
PV diagrams - part 2: Isothermal, isometric, adiabatic processes | MCAT | Khan Academy
PV Graph and First Law of Thermodynamics
FE Exam Review - Thermodynamics - PV Diagram
The First Law of Thermodynamics: Internal Energy, Heat, and Work
Temperature Entropy Diagram
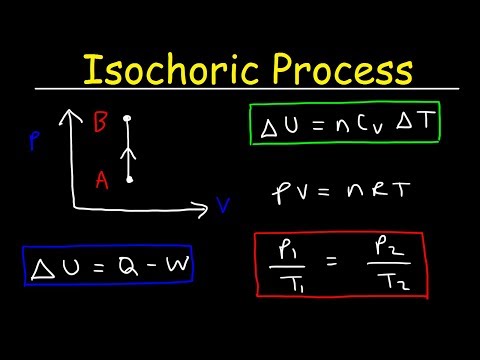
Isochoric Process Thermodynamics - Work, Heat & Internal Energy, PV Diagrams
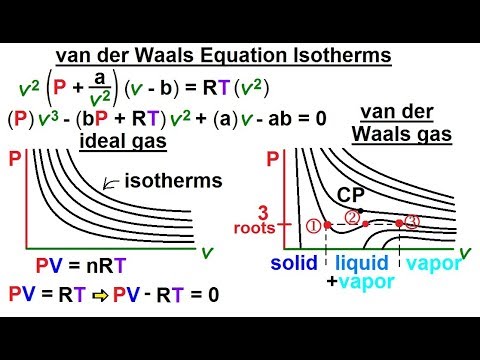
Physics - Thermodynamics 2: Ch 32.1 Def. and Terms (20 of 25) van der Waals Equation Isotherms
Internal Energy 1st Law of Thermodynamics U = w + q Heat and Work with PV Diagrams
First Law Thermodynamics, PV Diagram paths, Work
Комментарии
 0:07:53
0:07:53
 0:20:17
0:20:17
 0:11:54
0:11:54
 0:13:24
0:13:24
 0:03:47
0:03:47
 0:27:07
0:27:07
 0:02:53
0:02:53
 0:12:33
0:12:33
 0:33:04
0:33:04
 0:08:41
0:08:41
 0:10:44
0:10:44
 0:07:00
0:07:00
 0:03:13
0:03:13
 0:27:42
0:27:42
 0:11:55
0:11:55
 0:13:00
0:13:00
 0:08:13
0:08:13
 0:11:54
0:11:54
 0:05:44
0:05:44
 0:05:05
0:05:05
 0:11:01
0:11:01
 0:03:56
0:03:56
 0:11:22
0:11:22
 0:27:25
0:27:25