filmov
tv
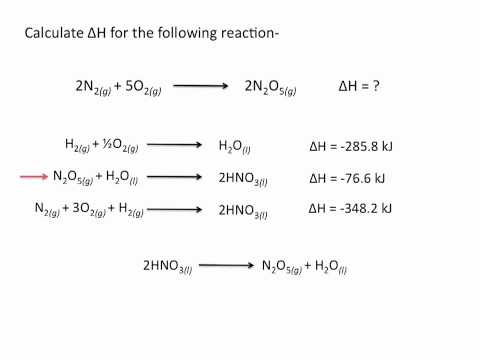
Practice Problem: Enthalpy of Combustion

Показать описание
Stoichiometry for combustion? You bet! If we know about moles, we can calculate energy changes. Give it a try!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Practice Problem: Enthalpy of Combustion
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Problems Chemistry
Hess's Law Problems & Enthalpy Change - Chemistry
Practice Problem On Calculating the Enthalpy of Gasoline Combustion
IIT/JEE Chemistry Practice #26: Enthalpy of Combustion
Hess's Law Example Problem
Hess's Law and Heats of Formation
Enthalpy of Combustion Calculation // HSC Chemistry
Thermodynamics | Thermochemistry | Standard heat of combustion #iit #neet #shorts #trending #quiz
The EASIEST Method For Solving Hess Cycles
Practice Problem On Calculating the Enthalpy of Gasoline Combustion
IB Chemistry Topic 5 Energetics 5.2 Hess's Law with enthalpy of formation and enthalpy of combu...
Thermochemistry Equations and Formulas With Practice Problems
Bond Energy Calculations & Enthalpy Change Problems, Basic Introduction, Chemistry
Hess's Law
Tricks to solve Thermochemistry problems easily | Enthalpy of formation combustion
Calculate Heat of Reaction at an Elevated Temperature
Heat / Enthalpy (∆H) Stoichiometry Practice Problems & Examples with Thermochemical Equations
Enthalpy Stoichiometry Part 2: How to Find Heat Released
Thermochemical Equations
Enthalpy of combustion of ethanol C0096
Practice Problem: Hess's Law
Hess's Law Common Test Question
Hess's Law - Chemistry Tutorial
Комментарии
 0:03:20
0:03:20
 0:16:42
0:16:42
 0:14:03
0:14:03
 0:00:48
0:00:48
 0:03:58
0:03:58
 0:04:11
0:04:11
 0:04:58
0:04:58
 0:12:55
0:12:55
 0:00:13
0:00:13
 0:13:46
0:13:46
 0:00:49
0:00:49
 0:07:40
0:07:40
 0:29:27
0:29:27
 0:11:39
0:11:39
 0:17:55
0:17:55
 0:17:40
0:17:40
 0:05:23
0:05:23
 0:07:18
0:07:18
 0:04:19
0:04:19
 0:12:47
0:12:47
 0:06:48
0:06:48
 0:06:59
0:06:59
 0:03:11
0:03:11
 0:11:23
0:11:23