filmov
tv
Thermochemistry Equations and Formulas With Practice Problems

Показать описание
This chemistry video tutorial provides a basic introduction into the equations and formulas that you need to solve common Thermochemistry practice problems.
First Law of Thermodynamics:
Thermochemistry Equations:
Internal Energy, Heat, and Work:
Thermochemical Equations:
Specific Vs Molar Heat Capacity:
________________________________
Basic Calorimetry Problems:
Final Temperature Calorimetry Problems:
Latent Heat of Fusion & Vaporization:
Coffee Cup Calorimeter:
More Calorimeter Problems:
__________________________________
Specific Heat Capacity Problems:
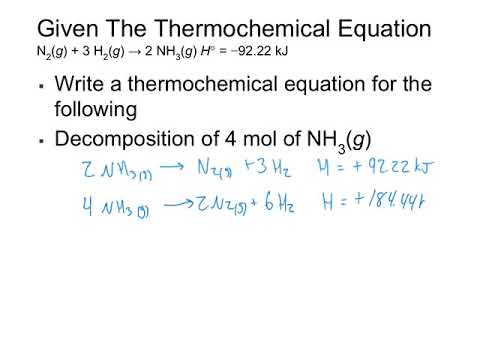
Hess Law Problems:
More Hess Law Problems:
Enthalpy of Formation & Heat Combustion:
Enthalpy Practice Problems:
__________________________________
Speed of Light, Frequency, & Wavelength:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
First Law of Thermodynamics:
Thermochemistry Equations:
Internal Energy, Heat, and Work:
Thermochemical Equations:
Specific Vs Molar Heat Capacity:
________________________________
Basic Calorimetry Problems:
Final Temperature Calorimetry Problems:
Latent Heat of Fusion & Vaporization:
Coffee Cup Calorimeter:
More Calorimeter Problems:
__________________________________
Specific Heat Capacity Problems:
Hess Law Problems:
More Hess Law Problems:
Enthalpy of Formation & Heat Combustion:
Enthalpy Practice Problems:
__________________________________
Speed of Light, Frequency, & Wavelength:
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:29:27
0:29:27
 0:12:47
0:12:47
 0:04:58
0:04:58
 0:06:45
0:06:45
 0:02:05
0:02:05
 0:14:03
0:14:03
 0:13:12
0:13:12
 0:11:27
0:11:27
 0:03:31
0:03:31
 0:05:31
0:05:31
 0:05:03
0:05:03
 0:08:37
0:08:37
 0:19:57
0:19:57
 0:03:57
0:03:57
 0:04:17
0:04:17
 0:06:21
0:06:21
 0:05:27
0:05:27
 0:15:34
0:15:34
 0:01:47
0:01:47
 0:04:39
0:04:39
 0:04:24
0:04:24
 0:03:34
0:03:34
 0:21:18
0:21:18
 0:00:35
0:00:35