filmov
tv
Electron Transport Chain ETC Made Easy

Показать описание
GET LECTURE HANDOUTS and other DOWNLOADABLE CONTENT FROM THIS VIDEO
SUPPORT US ON PATREON OR JOIN HERE ON YOUTUBE.
Electron Transport Chain ETC Made Easy
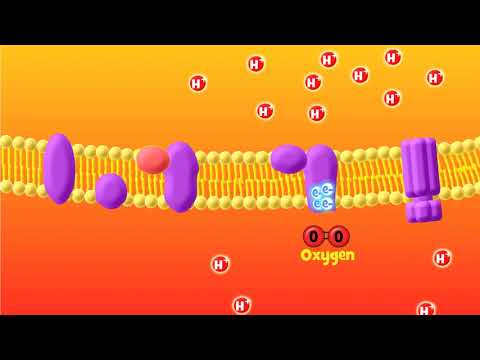
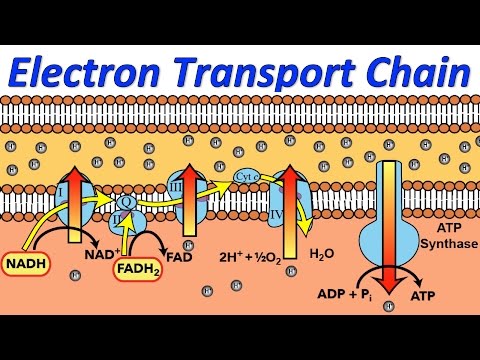
An electron transport chain (ETC) is a series of complexes that transfer electrons from electron donors to electron acceptors via redox (both reduction and oxidation occurring simultaneously) reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP), a molecule that stores energy chemically in the form of highly strained bonds. The molecules of the chain include peptides, enzymes (which are proteins or protein complexes), and others. The final acceptor of electrons in the electron transport chain during aerobic respiration is molecular oxygen although a variety of acceptors other than oxygen such as sulfate exist in anaerobic respiration.
In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient.
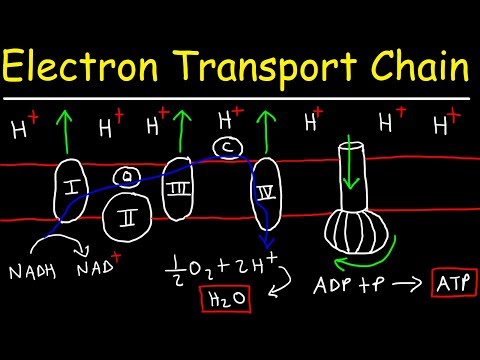
Complex I
In Complex I (NADH:ubiquinone oxidoreductase, NADH-CoQ reductase, or NADH dehydrogenase; EC 1.6.5.3), two electrons are removed from NADH and ultimately transferred to a lipid-soluble carrier, ubiquinone (Q). The reduced product, ubiquinol (QH2), freely diffuses within the membrane, and Complex I translocates four protons (H+) across the membrane, thus producing a proton gradient. Complex I is one of the main sites at which premature electron leakage to oxygen occurs, thus being one of the main sites of production of superoxide.
The pathway of electrons is as follows:
NADH is oxidized to NAD+, by reducing Flavin mononucleotide to FMNH2 in one two-electron step. FMNH2 is then oxidized in two one-electron steps, through a semiquinone intermediate. Each electron thus transfers from the FMNH2 to an Fe-S cluster, from the Fe-S cluster to ubiquinone (Q). Transfer of the first electron results in the free-radical (semiquinone) form of Q, and transfer of the second electron reduces the semiquinone form to the ubiquinol form, QH2. During this process, four protons are translocated from the mitochondrial matrix to the intermembrane space. [4] As the electrons become continuously oxidized and reduced throughout the complex an electron current is produced along the 180 Angstrom width of the complex within the membrane. This current powers the active transport of four protons to the intermembrane space per two electrons from NADH.
Complex II
In Complex II (succinate dehydrogenase or succinate-CoQ reductase; EC 1.3.5.1) additional electrons are delivered into the quinone pool (Q) originating from succinate and transferred (via flavin adenine dinucleotide (FAD)) to Q. Complex II consists of four protein subunits: succinate dehydrogenase, (SDHA); succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial, (SDHB); succinate dehydrogenase complex subunit C, (SDHC) and succinate dehydrogenase complex, subunit D, (SDHD). Other electron donors (e.g., fatty acids and glycerol 3-phosphate) also direct electrons into Q (via FAD). Complex 2 is a parallel electron transport pathway to complex 1, but unlike complex 1, no protons are transported to the intermembrane space in this pathway. Therefore, the pathway through complex 2 contributes less energy to the overall electron transport chain process.
Complex III
In Complex III (cytochrome bc1 complex or CoQH2-cytochrome c reductase; EC 1.10.2.2), the Q-cycle contributes to the proton gradient by an asymmetric absorption/release of protons. Two electrons are removed from QH2 at the QO site and sequentially transferred to two molecules of cytochrome c, a water-soluble electron carrier located within the intermembrane space. The two other electrons sequentially pass across the protein to the Qi site where the quinone part of ubiquinone is reduced to quinol. A proton gradient is formed by one quinol (2H+2e-) oxidations at the Qo site to form one quinone (2H+2e-) at the Qi site. (in total four protons are translocated: two protons reduce quinone to quinol and two protons are released from two ubiquinol molecules).
QH2 + 2 cytochrome c (FeIII) + 2 H+in → Q + 2 cytochrome c (FeII) + 4 H+out
When electron transfer is reduced (by a high membrane potential or respiratory inhibitors such as antimycin A), Complex III may leak electrons to molecular oxygen, resulting in superoxide formation.
-~-~~-~~~-~~-~-
CHECK OUT NEWEST VIDEO: "Nucleic acids - DNA and RNA structure "
-~-~~-~~~-~~-~-
SUPPORT US ON PATREON OR JOIN HERE ON YOUTUBE.
Electron Transport Chain ETC Made Easy
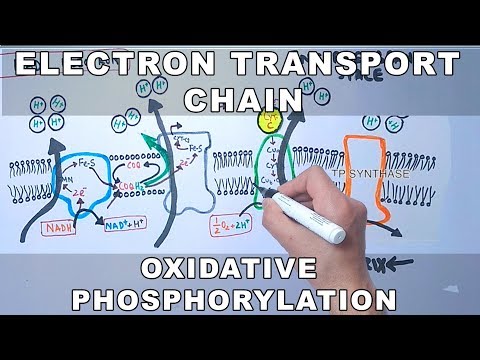
An electron transport chain (ETC) is a series of complexes that transfer electrons from electron donors to electron acceptors via redox (both reduction and oxidation occurring simultaneously) reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP), a molecule that stores energy chemically in the form of highly strained bonds. The molecules of the chain include peptides, enzymes (which are proteins or protein complexes), and others. The final acceptor of electrons in the electron transport chain during aerobic respiration is molecular oxygen although a variety of acceptors other than oxygen such as sulfate exist in anaerobic respiration.
In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient.
Complex I
In Complex I (NADH:ubiquinone oxidoreductase, NADH-CoQ reductase, or NADH dehydrogenase; EC 1.6.5.3), two electrons are removed from NADH and ultimately transferred to a lipid-soluble carrier, ubiquinone (Q). The reduced product, ubiquinol (QH2), freely diffuses within the membrane, and Complex I translocates four protons (H+) across the membrane, thus producing a proton gradient. Complex I is one of the main sites at which premature electron leakage to oxygen occurs, thus being one of the main sites of production of superoxide.
The pathway of electrons is as follows:
NADH is oxidized to NAD+, by reducing Flavin mononucleotide to FMNH2 in one two-electron step. FMNH2 is then oxidized in two one-electron steps, through a semiquinone intermediate. Each electron thus transfers from the FMNH2 to an Fe-S cluster, from the Fe-S cluster to ubiquinone (Q). Transfer of the first electron results in the free-radical (semiquinone) form of Q, and transfer of the second electron reduces the semiquinone form to the ubiquinol form, QH2. During this process, four protons are translocated from the mitochondrial matrix to the intermembrane space. [4] As the electrons become continuously oxidized and reduced throughout the complex an electron current is produced along the 180 Angstrom width of the complex within the membrane. This current powers the active transport of four protons to the intermembrane space per two electrons from NADH.
Complex II
In Complex II (succinate dehydrogenase or succinate-CoQ reductase; EC 1.3.5.1) additional electrons are delivered into the quinone pool (Q) originating from succinate and transferred (via flavin adenine dinucleotide (FAD)) to Q. Complex II consists of four protein subunits: succinate dehydrogenase, (SDHA); succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial, (SDHB); succinate dehydrogenase complex subunit C, (SDHC) and succinate dehydrogenase complex, subunit D, (SDHD). Other electron donors (e.g., fatty acids and glycerol 3-phosphate) also direct electrons into Q (via FAD). Complex 2 is a parallel electron transport pathway to complex 1, but unlike complex 1, no protons are transported to the intermembrane space in this pathway. Therefore, the pathway through complex 2 contributes less energy to the overall electron transport chain process.
Complex III
In Complex III (cytochrome bc1 complex or CoQH2-cytochrome c reductase; EC 1.10.2.2), the Q-cycle contributes to the proton gradient by an asymmetric absorption/release of protons. Two electrons are removed from QH2 at the QO site and sequentially transferred to two molecules of cytochrome c, a water-soluble electron carrier located within the intermembrane space. The two other electrons sequentially pass across the protein to the Qi site where the quinone part of ubiquinone is reduced to quinol. A proton gradient is formed by one quinol (2H+2e-) oxidations at the Qo site to form one quinone (2H+2e-) at the Qi site. (in total four protons are translocated: two protons reduce quinone to quinol and two protons are released from two ubiquinol molecules).
QH2 + 2 cytochrome c (FeIII) + 2 H+in → Q + 2 cytochrome c (FeII) + 4 H+out
When electron transfer is reduced (by a high membrane potential or respiratory inhibitors such as antimycin A), Complex III may leak electrons to molecular oxygen, resulting in superoxide formation.
-~-~~-~~~-~~-~-
CHECK OUT NEWEST VIDEO: "Nucleic acids - DNA and RNA structure "
-~-~~-~~~-~~-~-
Комментарии
 0:08:14
0:08:14
 0:04:53
0:04:53
 0:11:31
0:11:31
 0:11:06
0:11:06
 0:07:45
0:07:45
 0:29:22
0:29:22
 0:16:52
0:16:52
 0:04:45
0:04:45
 0:09:10
0:09:10
 0:16:04
0:16:04
 0:00:44
0:00:44
 0:31:07
0:31:07
 0:08:47
0:08:47
 0:04:37
0:04:37
 0:00:06
0:00:06
 0:06:01
0:06:01
 0:15:09
0:15:09
 0:13:19
0:13:19
 0:17:16
0:17:16
 0:04:39
0:04:39
 0:10:23
0:10:23
 0:05:55
0:05:55
 0:00:22
0:00:22
 0:08:43
0:08:43