filmov
tv
Formal Charge

Показать описание
This organic chemistry video tutorial explains how to calculate the formal of an atom in a molecule using a simple formula.
Valence Bond Theory - Full 33 Minute Video:
Formal Charge Review Notes:
Which Bond Is More Polar?
Condensed Structures to Skeletal Structures:
Functional Groups Review:
_________________________________
Primary, Secondary, & Tertiary Functional Groups:
Finding Lone Pairs Using Formal Charge:
Dipole Moment & Electronegativity:
Predicting Bond Angles:
Valence Bond Theory:
Hybridization of Atomic Orbitals:
_______________________________
Bond Strength and Bond Length:
Orbital Overlap and Bond Length:
Organic Chemistry PDF Worksheets:
Organic Chemistry Exam 1 Playlist:
Full-Length Videos and Worksheets:
Valence Bond Theory - Full 33 Minute Video:
Formal Charge Review Notes:
Which Bond Is More Polar?
Condensed Structures to Skeletal Structures:
Functional Groups Review:
_________________________________
Primary, Secondary, & Tertiary Functional Groups:
Finding Lone Pairs Using Formal Charge:
Dipole Moment & Electronegativity:
Predicting Bond Angles:
Valence Bond Theory:
Hybridization of Atomic Orbitals:
_______________________________
Bond Strength and Bond Length:
Orbital Overlap and Bond Length:
Organic Chemistry PDF Worksheets:
Organic Chemistry Exam 1 Playlist:
Full-Length Videos and Worksheets:
Formal Charge
How To Calculate The Formal Charge of an Atom - Chemistry
Resonance Structures/Assigning Formal Charge
Formal Charges Made Easy
Formal charge | Molecular and ionic compound structure and properties | AP Chemistry | Khan Academy
11. Formal Charge and Resonance
Trick to find Formal Charge
1.2 Formal Charges | Organic Chemistry
MISTRESS Drama Explodes as JAMES KENNEDY Faces DV Charges! | ALLY LEWBER Breaks Down!
Formal Charge Shortcut 🤯
Formal Charge Shortcut Organic Chemistry Basics Vid 4
Calculating NO3- Formal Charges: Calculating Formal Charges for NO3-
How to Draw Lewis Structures and Calculate Formal Charge
How to Find Formal Charges for your Organic Chemistry Class
Calculating O3 Formal Charges: Calculating Formal Charges for O3 (Ozone)
Formal Charge , Complete Topic in 6 Minutes
Formal Charge Practice Problems with Explanations
How to calculate Formal Charge without using formula #chemistry #class11 #neetchemistry #iitjee
Trick to predict formal charge|| formal charge calculation
S2.2.14 Formal charge (HL)
Formal charges guidelines #shorts #ochem
Formal Charges in Organic Chemistry
Lewis Structures and Formal Charges Practice Problems | Study Chemistry With Us
How To Draw Lewis Structures
Комментарии
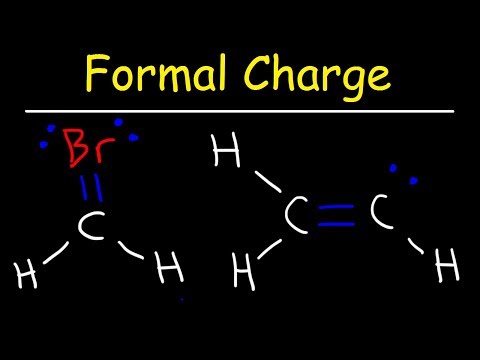 0:06:14
0:06:14
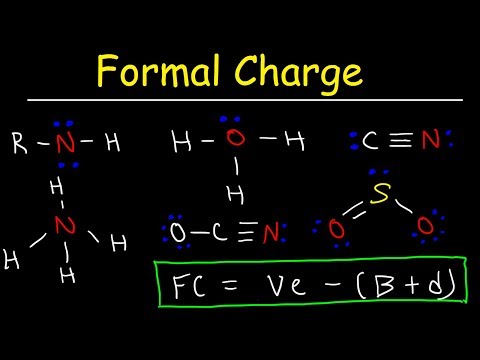 0:13:10
0:13:10
 0:12:32
0:12:32
 0:08:08
0:08:08
 0:07:03
0:07:03
 0:28:46
0:28:46
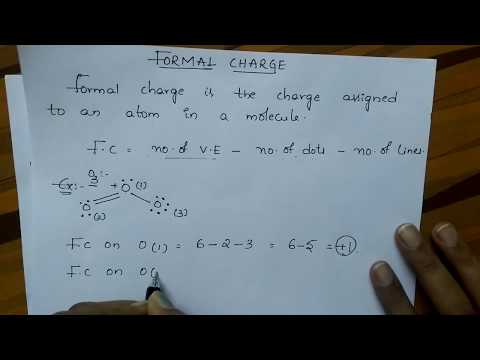 0:02:42
0:02:42
 0:07:08
0:07:08
 0:09:19
0:09:19
 0:00:55
0:00:55
 0:06:15
0:06:15
 0:02:24
0:02:24
 0:09:39
0:09:39
 0:04:14
0:04:14
 0:01:45
0:01:45
 0:06:29
0:06:29
 0:10:41
0:10:41
 0:00:53
0:00:53
 0:00:57
0:00:57
 0:05:46
0:05:46
 0:00:44
0:00:44
 0:13:03
0:13:03
 0:28:30
0:28:30
 0:11:50
0:11:50