filmov
tv
11. Formal Charge and Resonance

Показать описание
MIT 5.111 Principles of Chemical Science, Fall 2014
Instructor: Catherine Drennan
Radicals, expanded octets, and more, in this lecture about Lewis structures.
License: Creative Commons BY-NC-SA
Instructor: Catherine Drennan
Radicals, expanded octets, and more, in this lecture about Lewis structures.
License: Creative Commons BY-NC-SA
11. Formal Charge and Resonance
Resonance Structures/Assigning Formal Charge
Formal Charge
How To Calculate The Formal Charge of an Atom - Chemistry
Resonance Structures
Resonance Structures, Basic Introduction - How To Draw The Resonance Hybrid, Chemistry
Resonance and Formal Charge - AP Chem Unit 2, Topic 6
formal charge and resonance.mp4
Bond Order and Resonance Structures
Formal charge on carbon | Resonance and acid-base chemistry | Organic chemistry | Khan Academy
Formal Charge Shortcut 🤯
1.10 Practice: Formal Charge and Resonance
Drawing resonance structures when there’s a positive charge #organicchemistry #resonance
Formal Charge , Complete Topic in 6 Minutes
Lewis Sturctures: Resonance and Formal Charge
Drawing more resonance structures with multiple double bonds #organicchemistry
Formal charge | Molecular and ionic compound structure and properties | AP Chemistry | Khan Academy
Drawing Lewis Structures: Resonance Structures - Chemistry Tutorial
Lewis Dot Structures
How to calculate formal charge?
Formal Charge and Resonance
Formal Charge and Resonance - Explained
Polarity, Resonance, and Electron Pushing: Crash Course Organic Chemistry #10
Resonance Made Easy! Finding the Most Stable Resonance Structure - Organic Chemistry
Комментарии
 0:28:46
0:28:46
 0:12:32
0:12:32
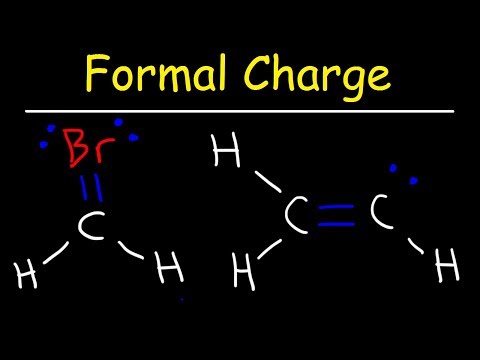 0:06:14
0:06:14
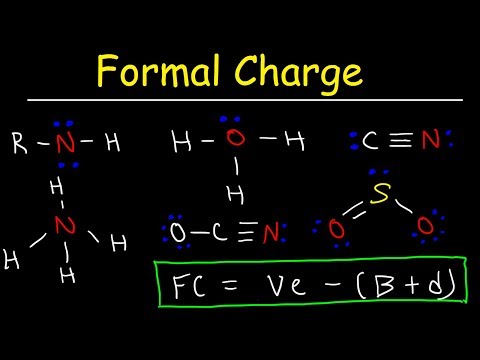 0:13:10
0:13:10
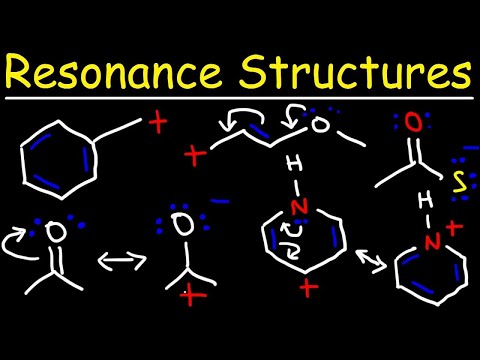 0:13:14
0:13:14
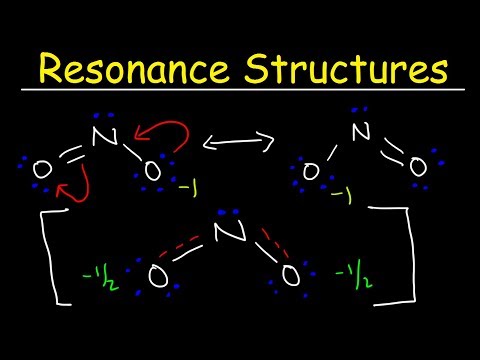 0:10:31
0:10:31
 0:12:13
0:12:13
 0:12:25
0:12:25
 0:05:25
0:05:25
 0:09:36
0:09:36
 0:00:55
0:00:55
 0:25:41
0:25:41
 0:00:26
0:00:26
 0:06:29
0:06:29
 0:21:39
0:21:39
 0:00:21
0:00:21
 0:07:03
0:07:03
 0:04:09
0:04:09
 0:04:41
0:04:41
 0:06:54
0:06:54
 0:01:28
0:01:28
 0:23:17
0:23:17
 0:11:46
0:11:46
 0:08:25
0:08:25