filmov
tv
How To Calculate The Formal Charge of an Atom - Chemistry
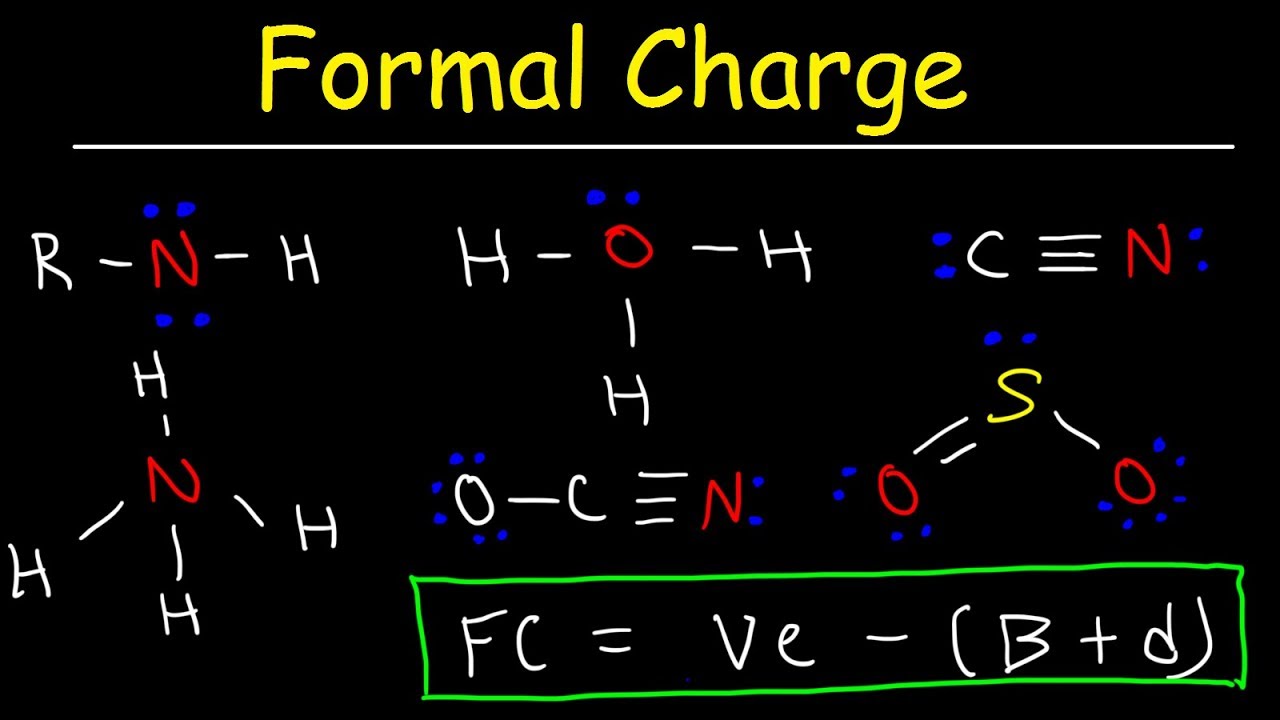
Показать описание
This chemistry video tutorial provides a basic introduction into how to calculate the formal charge of an atom or element in a lewis structure. This video is useful for students taking general chemistry or organic chemistry. This tutorial contains plenty of examples and practice problems so you can master using the formula / equation for calculating formal charge.
Molecular Geometry - Formula Sheet:
_____________________________
How To Draw Lewis Structures:
VSEPR Theory:
Molecular Geometry:
Lewis Dot Structures:
Lewis Structures of Ionic Compounds:
_________________________________
Octet Rule Exceptions:
Resonance Structures:
Polar and Nonpolar Molecules:
Formal Charge Calculations:
Lewis Structures - Mega Review:
________________________________
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
Hydrogen Bonding:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Formal Charge:
Molecular Geometry - Formula Sheet:
_____________________________
How To Draw Lewis Structures:
VSEPR Theory:
Molecular Geometry:
Lewis Dot Structures:
Lewis Structures of Ionic Compounds:
_________________________________
Octet Rule Exceptions:
Resonance Structures:
Polar and Nonpolar Molecules:
Formal Charge Calculations:
Lewis Structures - Mega Review:
________________________________
Hybridization of Atomic Orbitals:
Molecular Orbital Theory:
Dipole Dipole Forces of Attraction:
Hydrogen Bonding:
Unit Cell Chemistry:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Formal Charge:
Комментарии
 0:13:10
0:13:10
 0:06:14
0:06:14
 0:09:39
0:09:39
 0:03:41
0:03:41
 0:06:54
0:06:54
 0:04:14
0:04:14
 0:01:36
0:01:36
 0:02:08
0:02:08
 1:27:49
1:27:49
 0:07:08
0:07:08
 0:07:48
0:07:48
 0:02:24
0:02:24
 0:08:08
0:08:08
 0:01:15
0:01:15
 0:03:01
0:03:01
 0:07:03
0:07:03
 0:11:52
0:11:52
 0:01:33
0:01:33
 0:01:42
0:01:42
 0:02:34
0:02:34
 0:06:15
0:06:15
 0:01:28
0:01:28
 0:02:36
0:02:36
 0:01:46
0:01:46