filmov
tv
Quick Revision - pH titration curve calculations

Показать описание
Three past exam questions covering the pH titration curve topic.
Quick Revision - pH titration curves & indicators
Quick Revision - pH titration curve calculations
Acid-Base Titration
Acid Base Titration Curves
Acid Base Titration Curves - pH Calculations
Weak Acid / Strong Base Titration - All pH Calculations
AP Chem - Unit 8 Review - Acids and Bases in 10 Minutes - 2023
Strong Acid / Strong Base Titration Curve - All pH Calculations
Setting up and Performing a Titration
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry
Acid–base titrations | Chemical reactions | AP Chemistry | Khan Academy
Quick Revision - Titration Calculations
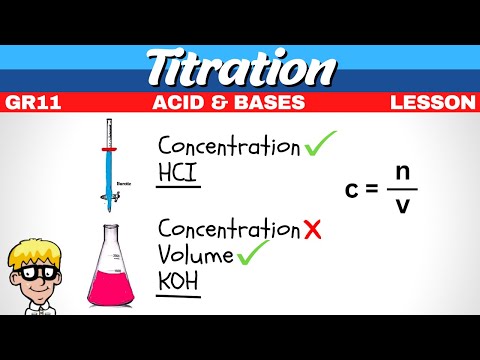
Titration Grade 11
17.2 Acid-Base Titrations and Titration Curves | General Chemistry
Quick Revision - Redox titrations
Titration curves and acid-base indicators | Chemistry | Khan Academy
How To Do Titrations | Chemical Calculations | Chemistry | FuseSchool
Titration | Acid Base Titration | Chemistry
Quick Revision - Further Titration Calculations
GCSE Chemistry - Acids and Bases #34
Back Titration Calculations – The Model Method [GCE A Level Chemistry]
Titrations | Titration Calculations | A level Chemistry | Explained
Acid-Base Equilibria and Buffer Solutions
Titration Method | Step-By-Step #experiment #chemistry
Комментарии
 0:08:16
0:08:16
 0:02:40
0:02:40
 0:08:02
0:08:02
 0:36:49
0:36:49
 0:18:52
0:18:52
 0:10:38
0:10:38
 0:13:29
0:13:29
 0:06:53
0:06:53
 0:18:35
0:18:35
 0:08:50
0:08:50
 0:04:50
0:04:50
 0:06:21
0:06:21
 0:28:50
0:28:50
 0:07:36
0:07:36
 0:07:10
0:07:10
 0:03:47
0:03:47
 0:10:49
0:10:49
 0:06:16
0:06:16
 0:04:37
0:04:37
 0:03:46
0:03:46
 0:28:46
0:28:46
 0:05:04
0:05:04
 0:00:56
0:00:56