filmov
tv
Strong Acid / Strong Base Titration Curve - All pH Calculations

Показать описание
-----
In this video, I calculate the pH at various points along a strong acid - strong base titration curve.
0:00 Calculating Equivalence Point Volume
1:49 Initial pH
2:34 pH Before the Equivalence Point (5mL)
5:46 pH Before the Equivalence Point (20mL)
7:34 pH at the Equivalence Point
9:19 pH After the Equivalence Point
11:12 Summary
How To Memorize The Strong Acids and Strong Bases
WORLDS STRONGEST ACID
GCSE Chemistry - The pH Scale & Strong vs Weak Acids (Higher Tier) #35
Strong Acid / Strong Base Titration Curve - All pH Calculations
Titration of Strong Acid With Strong Base
Strong acid–strong base reactions | Acids and bases | AP Chemistry | Khan Academy
Strong acid–strong base titrations | Acids and bases | AP Chemistry | Khan Academy
How to Memorize Strong Bases | Trick to Learn Strong Bases #shorts #reels #chemistry
calculate the pH of strong acid & strong bases###chemistry####
Strong acid strong base | pH calculations and auto ionization of water | grade 12 LS and GS
The strengths and weaknesses of acids and bases - George Zaidan and Charles Morton
Easy way to memorize the 7 strong acids and 6 strong bases
Strong Acid | Strong Base | Tricks to Learn Strong Acid & Strong Base | Chemistry By Kajal Ma&ap...
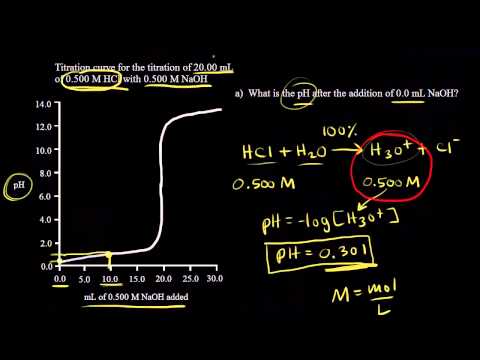
17.3a Strong Acid Strong Base Titrations pH Calculations | General Chemistry
Strong vs weak acids and bases (video)
How To Memorize The Strong Acids | Trick for Strong Acid & Weak Acid #shorts #reels #jee
How to Determine if Acid is Strong or Weak Shortcut w/ Examples and Practice Problems
Titration of a strong acid with a strong base | Chemistry | Khan Academy
Strong Acids |Examples of strong acids #shorts #science #acid #cbse #ssc
How to Memorize Strong Acids and Bases
Strong and weak acids/bases | Acids, bases, and salts | Chemistry | Khan Academy
How to Identify a Strong Base in Organic Chemistry
Strong acid|| weak acid|| strong base|| weak base with their chemical formula
Acids and Bases - Basic Introduction - Chemistry
Комментарии
 0:11:33
0:11:33
 0:00:31
0:00:31
 0:05:12
0:05:12
 0:13:29
0:13:29
 0:08:27
0:08:27
 0:09:29
0:09:29
 0:09:00
0:09:00
 0:00:45
0:00:45
 0:06:13
0:06:13
 0:26:55
0:26:55
 0:03:48
0:03:48
 0:01:39
0:01:39
 0:01:47
0:01:47
 0:16:23
0:16:23
 0:06:39
0:06:39
 0:00:49
0:00:49
 0:02:34
0:02:34
 0:10:12
0:10:12
 0:00:15
0:00:15
 0:01:51
0:01:51
 0:05:15
0:05:15
 0:03:52
0:03:52
 0:03:30
0:03:30
 0:58:42
0:58:42