filmov
tv
How to Find the Atomic Number for Atoms/Elements

Показать описание
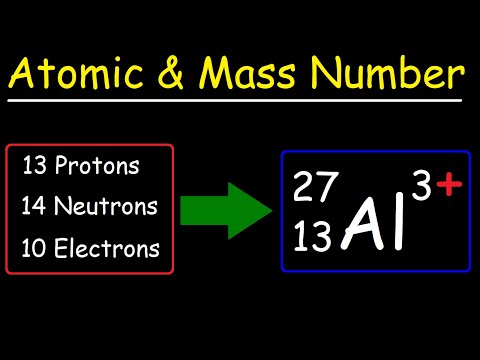
Being able to find the Atomic Number (also called the Proton Number) for elements is an essential chemistry skill. To find the Atomic Number all you need is a Periodic Table of the Elements.
For each element on the Periodic Table, the Atomic Number is the whole number above the element symbol. For example, the Atomic Number for Sodium (Na) is 11. For Hydrogen, H, it is 1.
The Atomic Number is defined as the number of protons that a particular atom has in its nucleus. For Sodium (Na) there the Atomic Number is 11. That means that there are 11 protons in the nucleus for an atom of Na. This is unique to Na, it is the only atom with 11 protons. Any more or less would not be Na.
For neutral elements (all the elements on the Periodic Table are neutral) the Atomic Number also equals the number of electrons for the atom. Since Na has an Atomic # of 11 it has 11 protons AND 11 electrons.
Helpful Resources:
For each element on the Periodic Table, the Atomic Number is the whole number above the element symbol. For example, the Atomic Number for Sodium (Na) is 11. For Hydrogen, H, it is 1.
The Atomic Number is defined as the number of protons that a particular atom has in its nucleus. For Sodium (Na) there the Atomic Number is 11. That means that there are 11 protons in the nucleus for an atom of Na. This is unique to Na, it is the only atom with 11 protons. Any more or less would not be Na.
For neutral elements (all the elements on the Periodic Table are neutral) the Atomic Number also equals the number of electrons for the atom. Since Na has an Atomic # of 11 it has 11 protons AND 11 electrons.
Helpful Resources:
How To Get Atomic Marker in Find The Markers Roblox 2023
Atomic Number, Mass Number, and Net Electric Charge
How To Get The *ATOMIC MARKER* In Roblox Find The Markers!
How to Find the Atomic Number for Atoms/Elements
How to get ATOMIC marker in FIND THE MARKERS Roblox [ UPDATED 2024 ]
How to Calculate Atomic Mass Practice Problems
Atomic Radius - Basic Introduction - Periodic Table Trends, Chemistry
The experiment that revealed the atomic world: Brownian Motion
What’s Happening to the Motorcycle Industry is Now Hitting the Auto Body World!
How To Calculate Relative Atomic Mass | Chemical Calculations | Chemistry | FuseSchool
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass Chemistry
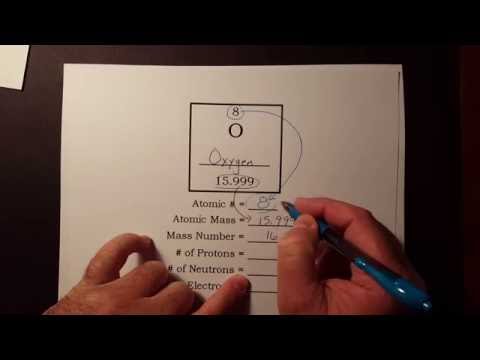
How to find the number of protons, neutrons, and electrons from the periodic table
Nuclide Symbols: Atomic Number, Mass Number, Ions, and Isotopes
Calculating atomic weight | Chemistry | Khan Academy
Easy trick to learn || Atomic mass|| 1 to 30 elements
Atomic Number, Mass Number, and Net Charge
Trick to Calculate Atomic Mass of first 20 Elements #shorts #reels #chemistry
Atomic Number and Mass Number.mov
Measuring Atomic Mass | Atoms and Molecules | Don't Memorise
Understanding Atomic Number and Atomic Mass
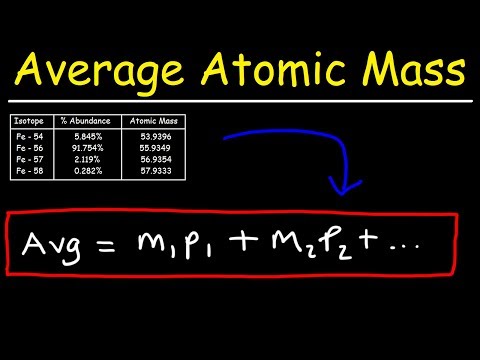
How To Calculate The Average Atomic Mass
Trick to Learn Atomic Masses of First 30 Elements of the Periodic Table
SECRET Atomic Samurai PLACE in The Strongest Battlegrounds
Atomic Mass: How to Calculate Isotope Abundance
Комментарии
 0:02:53
0:02:53
 0:11:41
0:11:41
 0:03:57
0:03:57
 0:03:07
0:03:07
 0:02:06
0:02:06
 0:06:11
0:06:11
 0:14:04
0:14:04
 0:12:26
0:12:26
 0:26:02
0:26:02
 0:03:48
0:03:48
 0:05:53
0:05:53
 0:07:41
0:07:41
 0:05:04
0:05:04
 0:04:26
0:04:26
 0:07:04
0:07:04
 0:06:27
0:06:27
 0:00:54
0:00:54
 0:06:13
0:06:13
 0:04:16
0:04:16
 0:02:24
0:02:24
 0:07:19
0:07:19
 0:06:47
0:06:47
 0:00:46
0:00:46
 0:11:49
0:11:49