filmov
tv
How To Calculate The Average Atomic Mass
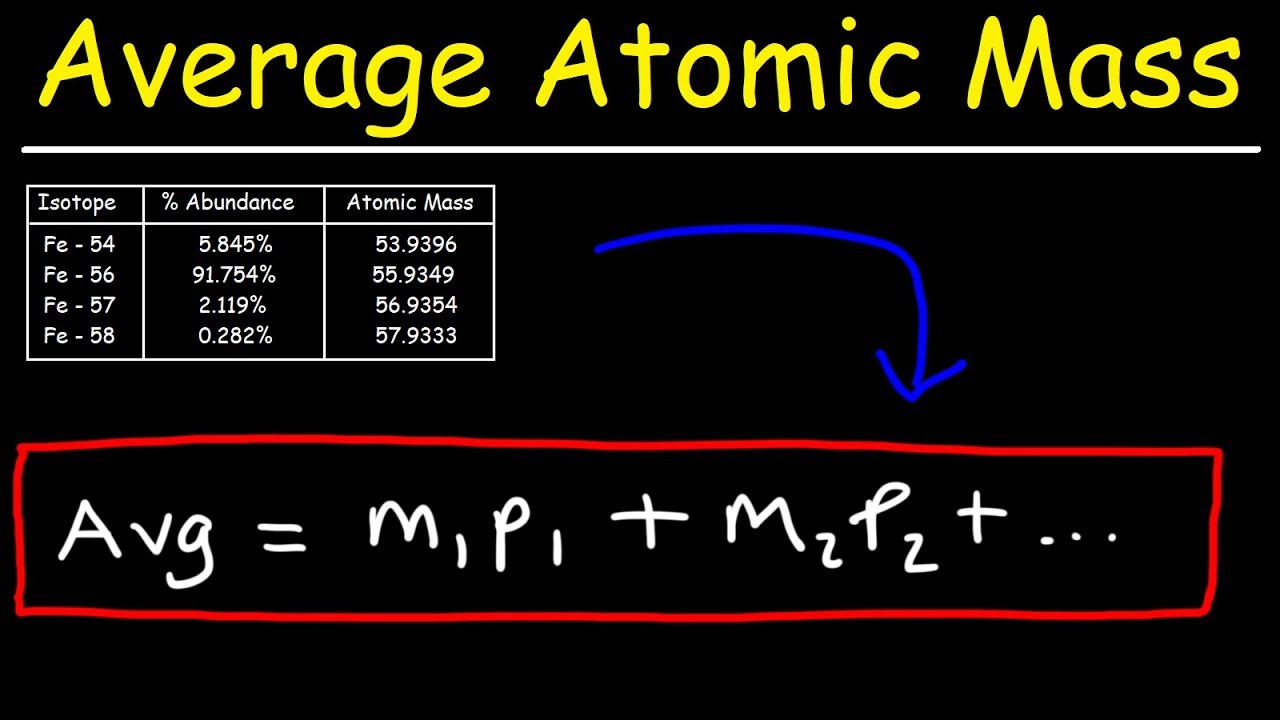
Показать описание
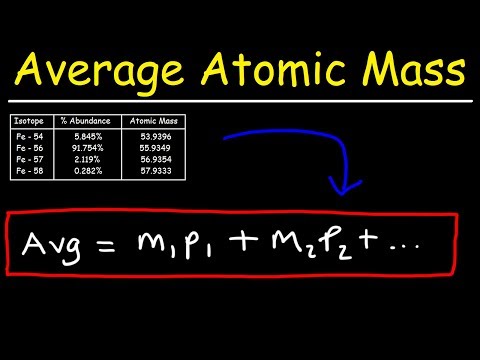
This chemistry video tutorial explains how to calculate the average atomic mass of an element given the percent abundance of each isotope.
Significant Figures Review:
Unit Conversion Problems:
Pure Substances & Mixtures:
Physical and Chemical Changes:
_________________________________
Atoms - Basic Introduction:
Cations and Anions Explained:
Diatomic Elements & Molecules:
Elements, Atoms, & Molecules:
Protons, Neutrons, & Electrons:
__________________________________
Average Atomic Mass:
What Are Isotopes?
Percent Abundance of Isotopes:
Ionic and Covalent Bonding:
Naming Molecular Compounds:
Writing Formulas - Ionic Compounds:
__________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Significant Figures Review:
Unit Conversion Problems:
Pure Substances & Mixtures:
Physical and Chemical Changes:
_________________________________
Atoms - Basic Introduction:
Cations and Anions Explained:
Diatomic Elements & Molecules:
Elements, Atoms, & Molecules:
Protons, Neutrons, & Electrons:
__________________________________
Average Atomic Mass:
What Are Isotopes?
Percent Abundance of Isotopes:
Ionic and Covalent Bonding:
Naming Molecular Compounds:
Writing Formulas - Ionic Compounds:
__________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:01:31
0:01:31
 0:03:01
0:03:01
 0:01:44
0:01:44
 0:03:42
0:03:42
 0:01:50
0:01:50
 0:03:44
0:03:44
 0:00:49
0:00:49
 0:03:01
0:03:01
 0:05:01
0:05:01
 0:00:51
0:00:51
 0:11:04
0:11:04
 0:02:52
0:02:52
 0:00:38
0:00:38
 0:05:39
0:05:39
 0:01:54
0:01:54
 0:01:53
0:01:53
 0:01:22
0:01:22
 0:02:34
0:02:34
 0:07:19
0:07:19
 0:02:16
0:02:16
 0:02:00
0:02:00
 0:01:55
0:01:55
 0:00:40
0:00:40
 0:01:08
0:01:08