filmov
tv
Understanding Atomic Number and Atomic Mass
Показать описание
Understanding Atomic Number and Atomic Mass
Atomic Number & Mass Number | Properties of Matter | Chemistry | FuseSchool
What's Inside an Atom? Protons, Electrons, and Neutrons!
Nuclide Symbols: Atomic Number, Mass Number, Ions, and Isotopes
Atomic Number & Mass Number | Properties of Matter | Chemistry | FuseSchool
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass Chemistry
Atomic Number | Atoms and Molecules | Don't Memorise
Atomic Number, Mass Number, and Net Electric Charge
Nuclei class 12 one shot || Nuclear Fusion and Fission || #hcverma #neet2025 #class12physics
Atoms for Kids | What is an atom? | Learn about atoms and molecules with activities and worksheets
Atom Explained in Simple Terms
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Quantum Numbers, Atomic Orbitals, and Electron Configurations
GCSE Physics - Atomic Structure, Isotopes & Electrons Shells #32
Atomic number, Atomic mass, Mass number: What's the difference?
History of Atomic Theory
GCSE Chemistry - Atoms & Ions #1
What Is An Atom And How Do We Know?
How small are atoms?
Atomic Structure And Electrons - Structure Of An Atom - What Are Atoms - Neutrons Protons Electrons
How To Calculate The Number of Protons, Neutrons, and Electrons - Chemistry
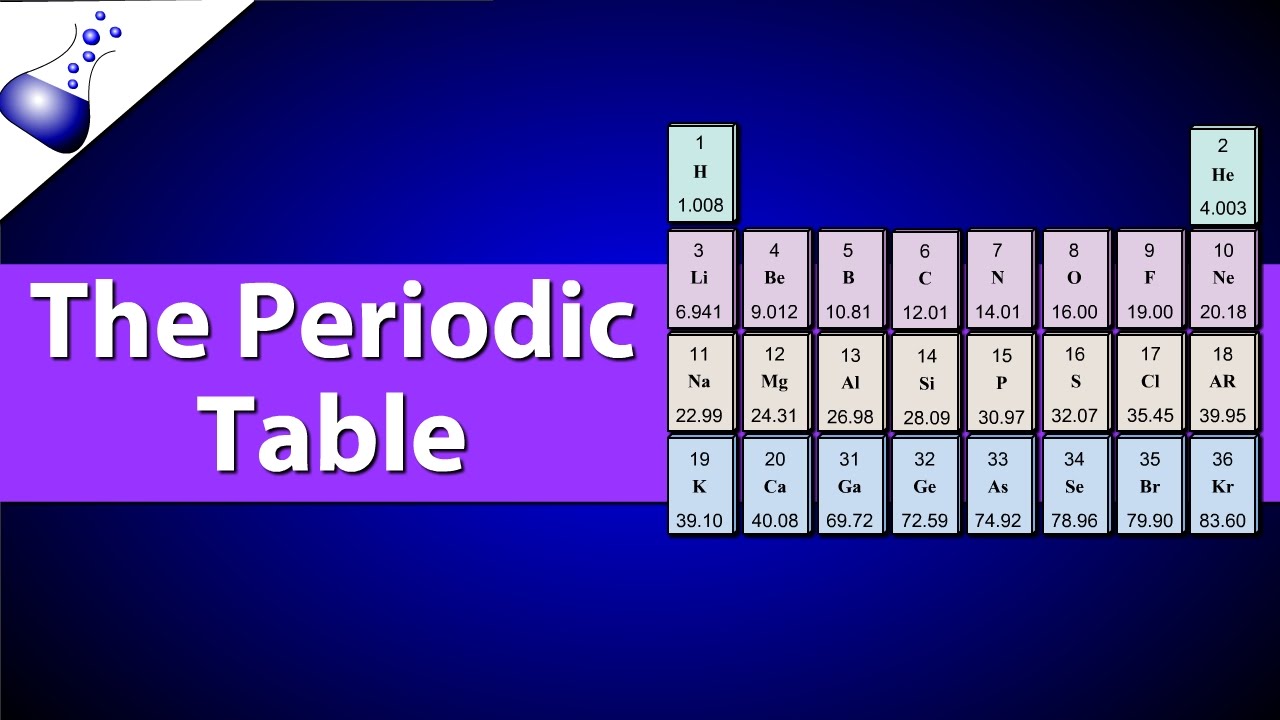
Periodic Table Explained: Introduction
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
How Small Is An Atom? Spoiler: Very Small.
Комментарии
 0:02:24
0:02:24
 0:03:23
0:03:23
 0:04:06
0:04:06
 0:05:04
0:05:04
 0:03:50
0:03:50
 0:05:53
0:05:53
 0:04:01
0:04:01
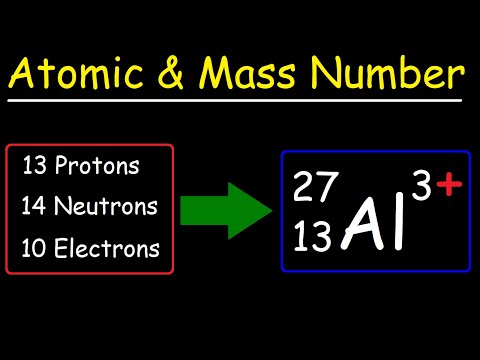 0:11:41
0:11:41
 1:35:33
1:35:33
 0:06:45
0:06:45
 0:01:44
0:01:44
 0:07:53
0:07:53
 0:08:42
0:08:42
 0:05:22
0:05:22
 0:06:38
0:06:38
 0:04:26
0:04:26
 0:07:20
0:07:20
 0:12:15
0:12:15
 0:00:48
0:00:48
 0:02:20
0:02:20
 0:13:12
0:13:12
 0:14:14
0:14:14
 0:11:19
0:11:19
 0:04:58
0:04:58