filmov
tv
Molarity

Показать описание
Learn about concentration, solutes, solvents, and molarity in this video!
Transcript
________________
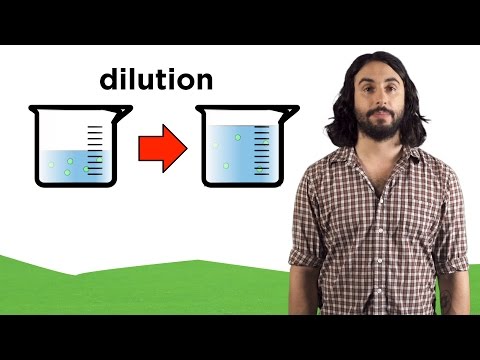
Have you ever noticed that Kool-aid at a summer camp tastes weaker than the kool-aid you might mix at home? Camps have a tendency to water down the drink mix and make a more diluted version than what the mix might call for to stretch their money.
Solutions with relatively more solvent and less solute are called dilute, while solutions with more solute and less solvent are concentrated.
In this diluted Kool aid, there aren’t very many particles of kool aid, the solute, relative to water, the solvent. The solute is the chemical that is dissolved and the solvent is the chemical that does the dissolving. In this case, it would probably taste better if you added more kool-aid powder and made a more concentrated solution. to measure concentration
chemists use molarity. molarity is the number of moles of solute dissolved in a solution and we use the capital letter M to represent it. You calculate molarity by taking the moles of solute and dividing by the liters of solution. Instead of writing units as moles per liter you just write a capital M for molar. Let’s try an example
What is the molarity of a solution with 0.70 mol solute in 250 mL of solution?
Molarity is moles divided by liters. the problem gives us 0.70 moles of solute, but the solution was given in milliliters, not liters. We need to convert milliliters into liters by dividing by 1000, then we can use that number in our calculation. and you get 2.8 molar. Solutions can have much lower concentrations than this, but you don’t often see concentrations higher than about 18 molar. Sometimes for shorthand, concentration will be written in brackets, like this. the brackets
Transcript
________________
Have you ever noticed that Kool-aid at a summer camp tastes weaker than the kool-aid you might mix at home? Camps have a tendency to water down the drink mix and make a more diluted version than what the mix might call for to stretch their money.
Solutions with relatively more solvent and less solute are called dilute, while solutions with more solute and less solvent are concentrated.
In this diluted Kool aid, there aren’t very many particles of kool aid, the solute, relative to water, the solvent. The solute is the chemical that is dissolved and the solvent is the chemical that does the dissolving. In this case, it would probably taste better if you added more kool-aid powder and made a more concentrated solution. to measure concentration
chemists use molarity. molarity is the number of moles of solute dissolved in a solution and we use the capital letter M to represent it. You calculate molarity by taking the moles of solute and dividing by the liters of solution. Instead of writing units as moles per liter you just write a capital M for molar. Let’s try an example
What is the molarity of a solution with 0.70 mol solute in 250 mL of solution?
Molarity is moles divided by liters. the problem gives us 0.70 moles of solute, but the solution was given in milliliters, not liters. We need to convert milliliters into liters by dividing by 1000, then we can use that number in our calculation. and you get 2.8 molar. Solutions can have much lower concentrations than this, but you don’t often see concentrations higher than about 18 molar. Sometimes for shorthand, concentration will be written in brackets, like this. the brackets
Комментарии
 0:08:46
0:08:46
 0:21:27
0:21:27
 0:31:25
0:31:25
 0:02:30
0:02:30
 0:05:41
0:05:41
 0:05:11
0:05:11
 0:04:54
0:04:54
 0:11:51
0:11:51
 0:00:55
0:00:55
 0:04:07
0:04:07
 0:05:03
0:05:03
 0:09:36
0:09:36
 0:08:20
0:08:20
 0:07:38
0:07:38
 0:15:50
0:15:50
 0:01:28
0:01:28
 0:16:12
0:16:12
 0:21:55
0:21:55
 0:07:08
0:07:08
 0:08:21
0:08:21
 0:02:01
0:02:01
 0:05:30
0:05:30
 0:04:32
0:04:32
 0:04:06
0:04:06