filmov
tv
Polyatomic Ions explained: Origin of Charge + Drawing Polyatomic Ion Lewis Dot Structures Tutorial
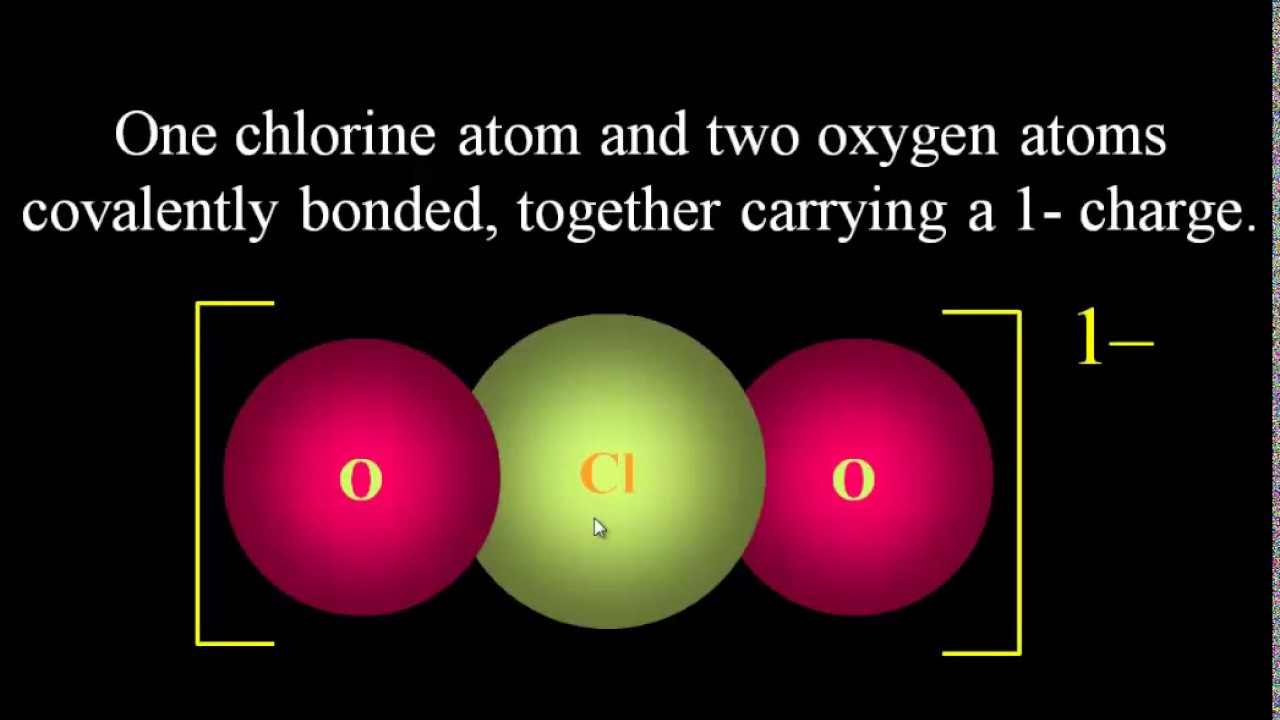
Показать описание
Crash course on using dot structures to illustrate why polyatomic ions acquire charge as well as understanding how to use charge when drawing Lewis dot structures of polyatomic ions
CC Academy videos are easy 101 crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments.
Check out our best lessons:
- Solution Stoichiometry Tutorial: How to use Molarity
- Stoichiometry
- Quantum Numbers
- Rutherford's Gold Foil Experiment, Explained
- Covalent Bonding Tutorial: Covalent vs. Ionic bonds
- Metallic Bonding and Metallic Properties Explained: Electron Sea Model
- Effective Nuclear Charge, Shielding, and Periodic Properties
- Electron Configuration Tutorial + How to Derive Configurations from Periodic Table
- Orbitals, the Basics: Atomic Orbital Tutorial — probability, shapes, energy
- Metric Prefix Conversions Tutorial
- Gas Law Practice Problems: Boyle's Law, Charles Law, Gay Lussac's, Combined Gas Law
—More on Lewis Dot Structures | Wiki—
"Lewis structures (also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.[1][2][3] A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article The Atom and the Molecule.[4] Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.
Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
Although main group elements of the second period and beyond usually react by gaining, losing, or sharing electrons until they have achieved a valence shell electron configuration with a full octet of (8) electrons, other elements obey different rules. Hydrogen (H) can only form bonds which share just two electrons, while transition metals often conform to a duodectet (12)[5] rule (e.g., compounds such as the permanganate ion)."
Wikipedia contributors. "Lewis structure." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 8 Jun. 2016. Web. 2 Jul. 2016.
—More on Polyatomic Ions | Wiki—
"A polyatomic ion, also known as a molecular ion, is a charged chemical species (ion) composed of two or more atoms covalently bonded or of a metal complex that can be considered to be acting as a single unit. The prefix poly- means "many," in Greek, but even ions of two atoms are commonly referred to as polyatomic. In older literature, a polyatomic ion is also referred to as a radical, and less commonly, as a radical group. In contemporary usage, the term radical refers to free radicals that are (not necessarily charged) species with an unpaired electron.
An example of a polyatomic ion is the hydroxide ion; consisting of one oxygen atom and one hydrogen atom, hydroxide has a charge of −1. Its chemical formula is OH−. An ammonium ion is made up of one nitrogen atom and four hydrogen atoms: it has a charge of +1, and its chemical formula is NH+
4.
Polyatomic ions are often useful in the context of acid-base chemistry or in the formation of salts. A polyatomic ion can often be considered as the conjugate acid/base of a neutral molecule. For example, the conjugate base of sulfuric acid (H2SO4) is the polyatomic hydrogen sulfate anion (HSO−
4). The removal of another hydrogen ion yields the sulfate anion (SO2−
4).
Wikipedia contributors. "Polyatomic ion." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 10 Jun. 2016. Web. 2 Jul. 2016.
CC Academy videos are easy 101 crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments.
Check out our best lessons:
- Solution Stoichiometry Tutorial: How to use Molarity
- Stoichiometry
- Quantum Numbers
- Rutherford's Gold Foil Experiment, Explained
- Covalent Bonding Tutorial: Covalent vs. Ionic bonds
- Metallic Bonding and Metallic Properties Explained: Electron Sea Model
- Effective Nuclear Charge, Shielding, and Periodic Properties
- Electron Configuration Tutorial + How to Derive Configurations from Periodic Table
- Orbitals, the Basics: Atomic Orbital Tutorial — probability, shapes, energy
- Metric Prefix Conversions Tutorial
- Gas Law Practice Problems: Boyle's Law, Charles Law, Gay Lussac's, Combined Gas Law
—More on Lewis Dot Structures | Wiki—
"Lewis structures (also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.[1][2][3] A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article The Atom and the Molecule.[4] Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.
Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms.
Although main group elements of the second period and beyond usually react by gaining, losing, or sharing electrons until they have achieved a valence shell electron configuration with a full octet of (8) electrons, other elements obey different rules. Hydrogen (H) can only form bonds which share just two electrons, while transition metals often conform to a duodectet (12)[5] rule (e.g., compounds such as the permanganate ion)."
Wikipedia contributors. "Lewis structure." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 8 Jun. 2016. Web. 2 Jul. 2016.
—More on Polyatomic Ions | Wiki—
"A polyatomic ion, also known as a molecular ion, is a charged chemical species (ion) composed of two or more atoms covalently bonded or of a metal complex that can be considered to be acting as a single unit. The prefix poly- means "many," in Greek, but even ions of two atoms are commonly referred to as polyatomic. In older literature, a polyatomic ion is also referred to as a radical, and less commonly, as a radical group. In contemporary usage, the term radical refers to free radicals that are (not necessarily charged) species with an unpaired electron.
An example of a polyatomic ion is the hydroxide ion; consisting of one oxygen atom and one hydrogen atom, hydroxide has a charge of −1. Its chemical formula is OH−. An ammonium ion is made up of one nitrogen atom and four hydrogen atoms: it has a charge of +1, and its chemical formula is NH+
4.
Polyatomic ions are often useful in the context of acid-base chemistry or in the formation of salts. A polyatomic ion can often be considered as the conjugate acid/base of a neutral molecule. For example, the conjugate base of sulfuric acid (H2SO4) is the polyatomic hydrogen sulfate anion (HSO−
4). The removal of another hydrogen ion yields the sulfate anion (SO2−
4).
Wikipedia contributors. "Polyatomic ion." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 10 Jun. 2016. Web. 2 Jul. 2016.
Комментарии
 0:05:52
0:05:52
 0:06:58
0:06:58
 0:01:00
0:01:00
 0:02:04
0:02:04
 0:29:45
0:29:45
 0:07:41
0:07:41
 0:00:34
0:00:34
 0:02:45
0:02:45
 0:17:21
0:17:21
 0:01:12
0:01:12
 0:01:19
0:01:19
 0:08:02
0:08:02
 0:03:21
0:03:21
 0:03:02
0:03:02
 0:09:58
0:09:58
 0:03:49
0:03:49
 0:01:33
0:01:33
 0:08:01
0:08:01
 0:08:42
0:08:42
 0:14:44
0:14:44
 0:05:48
0:05:48
 0:00:26
0:00:26
 0:05:16
0:05:16
 0:01:26
0:01:26