filmov
tv
Physical Chemistry - Introduction
Показать описание
Short lecture introducing physical chemistry.
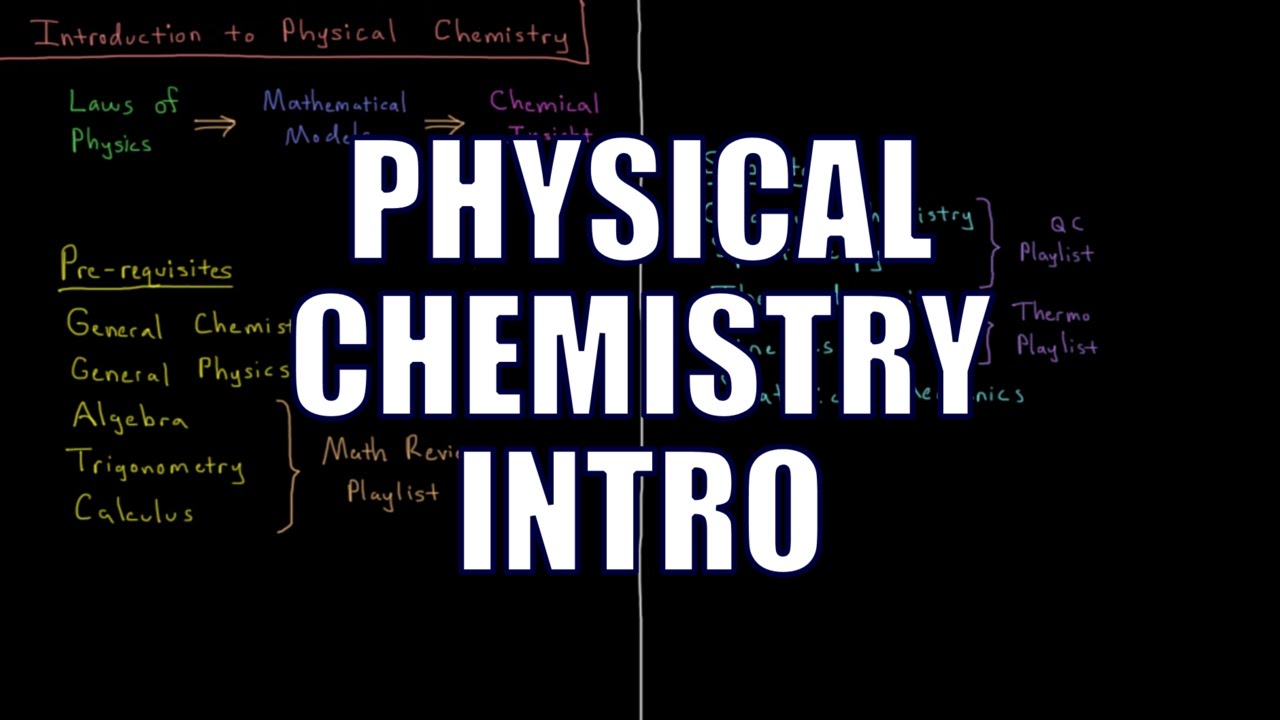
Physical chemistry is the use of the laws of physics to develop insight into chemical systems. Physical chemistry it typically studied for 2 or 3 semesters by undergraduate chemistry students. Prerequisites include general chemistry, general physics, algebra, trigonometry, and calculus. Subdivisions of physical chemistry include quantum chemistry, spectroscopy, thermodynamics, kinetics, and statistical mechanics.
This channel includes courses on Quantum Chemistry and Spectroscopy, Chemical Thermodynamics and Kinetics, Computational Chemistry, and PChem Math, as well as review videos within each chapter and course.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
Physical chemistry is the use of the laws of physics to develop insight into chemical systems. Physical chemistry it typically studied for 2 or 3 semesters by undergraduate chemistry students. Prerequisites include general chemistry, general physics, algebra, trigonometry, and calculus. Subdivisions of physical chemistry include quantum chemistry, spectroscopy, thermodynamics, kinetics, and statistical mechanics.
This channel includes courses on Quantum Chemistry and Spectroscopy, Chemical Thermodynamics and Kinetics, Computational Chemistry, and PChem Math, as well as review videos within each chapter and course.
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
Комментарии
 0:04:43
0:04:43
 0:11:57
0:11:57
 0:18:49
0:18:49
 0:11:38
0:11:38
 0:01:45
0:01:45
 0:11:56
0:11:56
 0:02:21
0:02:21
 11:59:56
11:59:56
 1:00:01
1:00:01
 0:11:27
0:11:27
 0:01:31
0:01:31
 0:09:54
0:09:54
 0:03:03
0:03:03
 0:02:36
0:02:36
 0:06:30
0:06:30
 0:07:45
0:07:45
 0:00:48
0:00:48
 0:11:07
0:11:07
 0:05:30
0:05:30
 0:00:37
0:00:37
 0:02:29
0:02:29
 0:11:16
0:11:16
 0:12:32
0:12:32
 0:05:02
0:05:02