filmov
tv
Understanding Molecular Orbital Theory
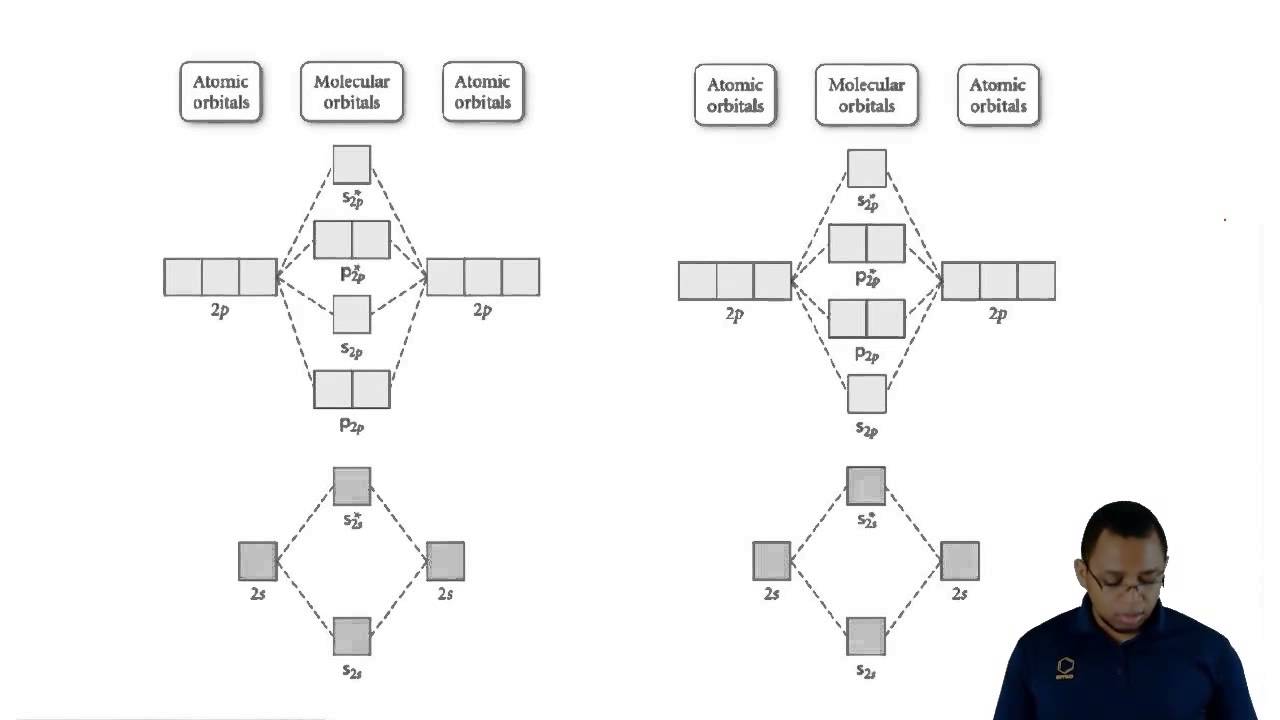
Показать описание
Explore Channels, available in Pearson+, and access thousands of videos with bite-sized lessons in multiple college courses. The videos are hand-picked, and led by experts, to help you learn faster and easier. Understand tricky concepts, quiz yourself with practice questions, download worksheets to follow along with the lessons and stay on top of your studies. Plus, experts are available for Q&A. Whether you want it paired with your eTextbook in Pearson+, or watch videos on your own time to go from "huh?" to "aha!" in class, Channels is available to you anytime you need it. It's reimagined learning, designed for your learning style.
---------------
----------------
SOCIAL:
----------------
#PearsonPlus #ThisIsYouLearning #studyvideo #StudyHelp #studying #studytutorial #eTextbooks #StudyToolsForLess #MoreBooksFewerBucks #BingeWatchGoodGrades
---------------
----------------
SOCIAL:
----------------
#PearsonPlus #ThisIsYouLearning #studyvideo #StudyHelp #studying #studytutorial #eTextbooks #StudyToolsForLess #MoreBooksFewerBucks #BingeWatchGoodGrades
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
Molecular Orbital Theory - Bonding & Antibonding MO - Bond Order
Understanding Molecular Orbital Theory
How atoms REALLY make molecules!
Drawing Molecular Orbital Diagrams
Molecular Orbital MO Theory Simplified for Sigma and Pi Bonds
Orbitals: Crash Course Chemistry #25
Molecular Orbital Theory | Chemistry
Atomic Structure | CUET PG PYQ Series | Inorganic Chemistry | Sonia Mam Chemistry
Bonding and Antibonding Molecular Orbitals
9.5 Molecular Orbital Theory | General Chemistry
Molecular Orbital (MO) Diagram for O2(-)
MO Theory 1: Molecular Orbital Theory Basics (for Organic Chemistry)
Examples of s-p Mixing in Molecular Orbital Theory
12. Molecular Orbitals (Intro to Solid-State Chemistry)
CHEMISTRY 101: Molecular Orbital Theory, Bond order, bond strength, magnetic properties
what is molecular orbital theory| class 11 3d animation
13. Molecular Orbital Theory
Molecular Orbital Theory (MOT) , Quick Revision in 5 Minutes
Ch#3 |Lec#8 | Molecular Orbital Theory (MOT) #mot#theory #chemistry 1
O=O is WRONG!
1.4 Molecular Orbital Theory | Organic Chemistry
11 Chap 4 | Chemical Bonding 10 | Molecular Orbital Theory IIT JEE NEET || MOT Part I Introduction |
Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
Комментарии
 0:07:54
0:07:54
 0:21:36
0:21:36
 0:05:36
0:05:36
 0:26:07
0:26:07
 0:11:05
0:11:05
 0:13:19
0:13:19
 0:10:52
0:10:52
 0:19:13
0:19:13
 0:29:52
0:29:52
 0:07:46
0:07:46
 0:45:53
0:45:53
 0:04:59
0:04:59
 0:03:21
0:03:21
 0:09:49
0:09:49
 0:48:53
0:48:53
 0:05:51
0:05:51
 0:00:34
0:00:34
 1:05:37
1:05:37
 0:05:48
0:05:48
 0:19:00
0:19:00
 0:00:50
0:00:50
 0:22:47
0:22:47
 0:29:14
0:29:14
 0:10:55
0:10:55