filmov
tv
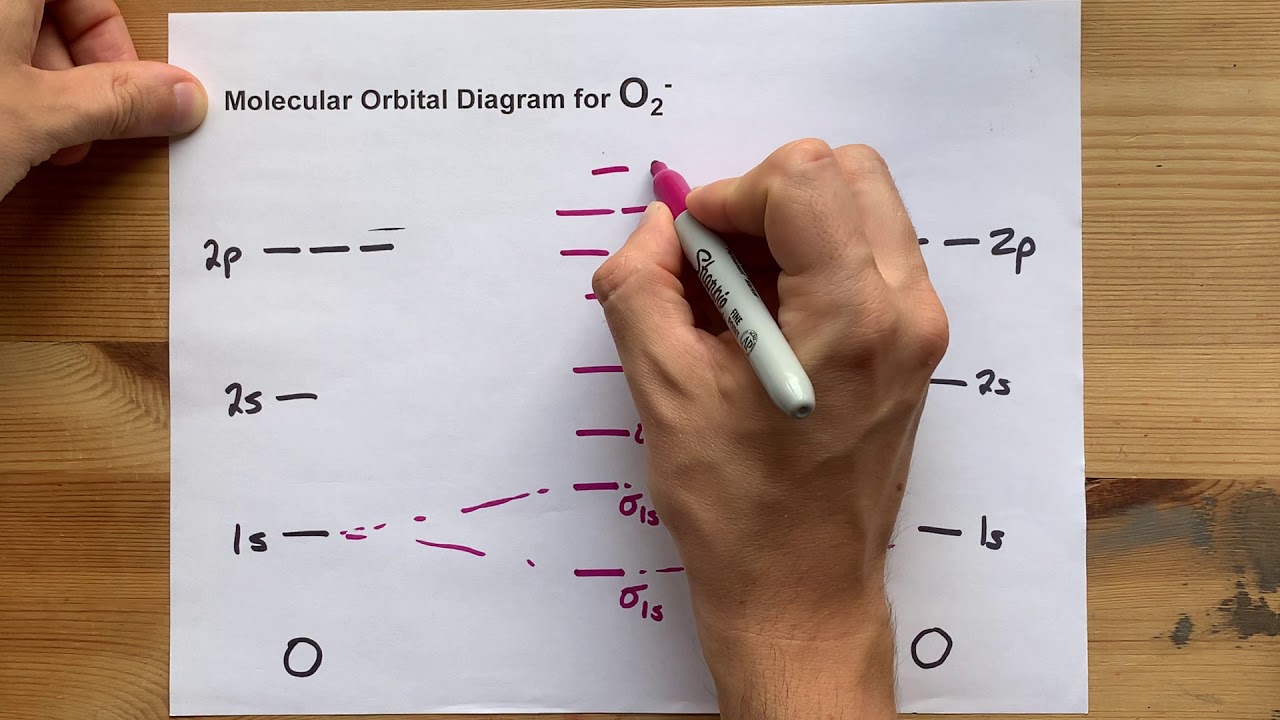
Molecular Orbital (MO) Diagram for O2(-)
Показать описание
When two oxygen atoms overlap, the sigma(2p) molecular orbital is LOWER in energy than the pi(2p) orbitals. This different from Nitrogen, where it's the other way around.
O2(-1) is just like regular molecular oxygen, but the extra electron causes its bond to be unstable. Its bond order is 1.5
O2(-1) is just like regular molecular oxygen, but the extra electron causes its bond to be unstable. Its bond order is 1.5
Molecular Orbital (MO) Diagram for O2(-)
Drawing Molecular Orbital Diagrams
Molecular Orbital Theory - Bonding & Antibonding MO - Bond Order
Molecular Orbital MO Theory Simplified for Sigma and Pi Bonds
Constructing the HF molecular orbital energy level diagram
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
Molecular Orbital (MO) Diagram for N2(-)
9.5 Molecular Orbital Theory | General Chemistry
Molecular Orbital (MO) Diagram for O2(2+)
Molecular Orbital (MO) Diagram for C2(2-)
CHEMISTRY 101: Molecular Orbital Theory, Bond order, bond strength, magnetic properties
Molecular orbital (MO) diagrams in organic chemistry
Examples of s-p Mixing in Molecular Orbital Theory
Molecular Orbital Diagrams Heteronuclear Diatomic HF
Molecular Orbital Theory | mo diagram of NO | mo diagram of CO |mo diagram of CN | mo diagram of HF
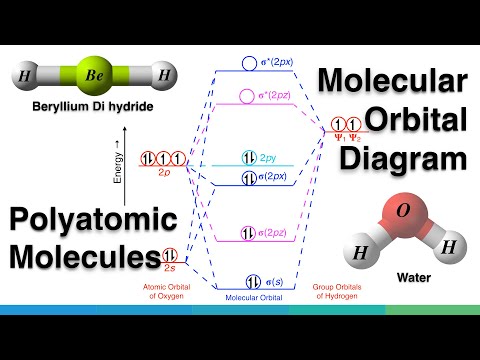
Molecular Orbital (MO) Diagram of Polyatomic molecules Beryllium dihydride (BeH2) and Water (H2O)
Molecular orbital theory. Heteronuclear diatomics. CO
molecular orbital theory | mo diagram of CO | mo diagram of NO | mo diagram of CN | mo diagram of HF
Molecular orbital diagram of NO #chemistryaplus #ytshorts
Molecular Orbital Diagram 👍👍✍️✍️
Molecular Orbital (MO) Diagram for O2(2-)
Construction of MO Diagrams for Simple Polyatomic Molecules
Molecular Orbital (MO) Diagram for F2(2+)
Draw the molecular orbital (MO) electron diagram for the H2- molecular ion
Комментарии
 0:04:59
0:04:59
 0:11:05
0:11:05
 0:21:36
0:21:36
 0:13:19
0:13:19
 0:02:03
0:02:03
 0:07:54
0:07:54
 0:04:11
0:04:11
 0:45:53
0:45:53
 0:04:15
0:04:15
 0:04:01
0:04:01
 0:05:51
0:05:51
 0:24:19
0:24:19
 0:09:49
0:09:49
 0:03:13
0:03:13
 0:12:19
0:12:19
 0:14:05
0:14:05
 0:07:35
0:07:35
 0:05:40
0:05:40
 0:00:08
0:00:08
 0:00:15
0:00:15
 0:04:40
0:04:40
 0:37:51
0:37:51
 0:04:10
0:04:10
 0:02:05
0:02:05