filmov
tv
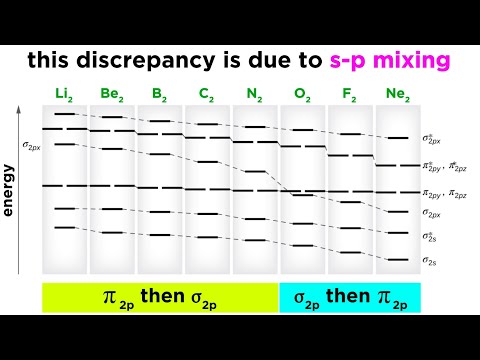
Examples of s-p Mixing in Molecular Orbital Theory

Показать описание
Admittedly, my prior tutorial on MO theory was a little confusing, and had some errors. I wanted to make things right, so here's another one! This will clarify some of the basic concepts, and will also extend them to discuss a new concept, s-p mixing. Let's dive right in!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Examples of s-p Mixing in Molecular Orbital Theory
Origins of s-p mixing
Example MO Diagram with s p Mixing
8.48 | Predict whether the MO diagram for S2 would show s-p mixing or not.
QM interpretation of sp mixing
S P Mixing of Molecular Orbitals
Chem 61 Lecture 8.L s-p Mixing in MO diagrams
Letest model of CO with sp mixing ✍️👍
iPad Music and Roland P6….having fun
#S-P #Orbital Mixing #MO Diagram
S-P Mixing and without S-P mixing ki in Molecular Orbital Theory (MOT)
MOT S-P Mixing by PMS Sir
s-p mixing in MOT
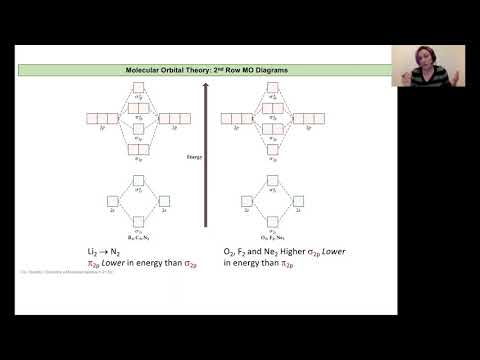
Molecular Orbital Theory and sp Orbital Mixing
How s-p mixing affect the Orbital Energy
Valence Bond Theory, Hybrid Orbitals, and Molecular Orbital Theory
L13B MO Diagram Example and Orbital Mixing
Molecular Orbital Theory - Bonding & Antibonding MO - Bond Order
Hybridization of Atomic Orbitals | SP, SP2, SP3 Hybridization of Carbon
Hybridization: mixing of s, p, and d orbitals
What is entropy? - Jeff Phillips
MO Diagrams of Homonuclear Diatomics No sp Mixing
EQ Explained in 10 Minutes ... or it's free
Hot🔥 + Cold❄️ || Emoji Mixing Satisfying Art #creativeart #satisfying
Комментарии
 0:09:49
0:09:49
 0:02:35
0:02:35
 0:17:03
0:17:03
 0:03:45
0:03:45
 0:26:41
0:26:41
 0:08:22
0:08:22
 0:13:18
0:13:18
 0:00:16
0:00:16
 0:53:30
0:53:30
 0:06:45
0:06:45
 0:27:40
0:27:40
 0:20:36
0:20:36
 0:16:36
0:16:36
 0:11:44
0:11:44
 0:05:18
0:05:18
 0:07:54
0:07:54
 0:14:17
0:14:17
 0:21:36
0:21:36
 0:13:48
0:13:48
 0:19:51
0:19:51
 0:05:20
0:05:20
 0:17:45
0:17:45
 0:09:47
0:09:47
 0:00:35
0:00:35