filmov
tv
Electron Configuration Sub shells and orbitals

Показать описание
A look at the electron configuration needed for A Level Chemistry. An introduction to the orbitals and how to work out the electron configuration from using the periodic table. A crucial part of Chemistry. Enjoy!
Electron Configuration - Basic introduction
Electron Configuration - Electron Subshells - Suborbitals - s, p, d, f - Orbitals - Chemistry
Shells, Subshells, and Orbitals - BIOLOGY/CHEMISTRY EP5
Electron Configuration Sub shells and orbitals
Quantum Numbers, Atomic Orbitals, and Electron Configurations
What are Shells, Subshells, and Orbitals? | Chemistry
PSC CHEMISTRY ELECTRONIC CONFIGURATION
How to Write the Electron Configuration for an Element in Each Block
Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy
Electron Configuration Diagrams | Properties of Matter | Chemistry | FuseSchool
Electron Configuration
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
SPDF Electronic Configuration Trick | Super trick
Introduction to electron configurations | AP Chemistry | Khan Academy
Subshell electron configuration|| For SSLC students and Kerala PSC aspirants
SSLC Chemistry Subshell Electronic Configuration | Chemistry Chapter 1 Important Topic | Eduport
Subshell Electronic Configuration | SSLC Chemistry | Chapter 1 | Important Question | Exam Winner
Complete Electronic Configuration |Aufbau Principle | Hund's Rule | Pauli Exclusion Principle
How to Write an Electron Configuration #chemistry #shorts #science #education #homework
Inside Atoms: Electron Shells and Valence Electron
How To do Electronic Configuration || Atomic Structure 08 || Electronic Configuration ||spdf
How small are atoms?
A Level Chemistry Revision 'Electron Configuration and the Periodic Table'
electronic configuration (subshell) 1 to 30 ✍️✍️
Комментарии
 0:10:19
0:10:19
 0:20:21
0:20:21
 0:09:23
0:09:23
 0:10:56
0:10:56
 0:08:42
0:08:42
 0:06:00
0:06:00
 0:40:21
0:40:21
 0:07:23
0:07:23
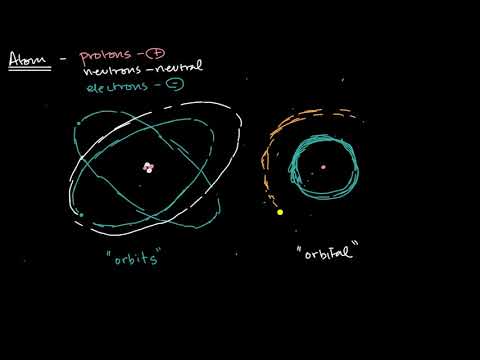 0:09:41
0:09:41
 0:04:59
0:04:59
 0:10:17
0:10:17
 0:11:19
0:11:19
 0:04:36
0:04:36
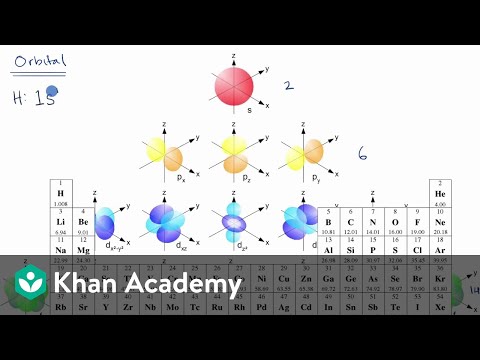 0:05:08
0:05:08
 0:22:25
0:22:25
 0:07:53
0:07:53
 0:09:28
0:09:28
 0:12:13
0:12:13
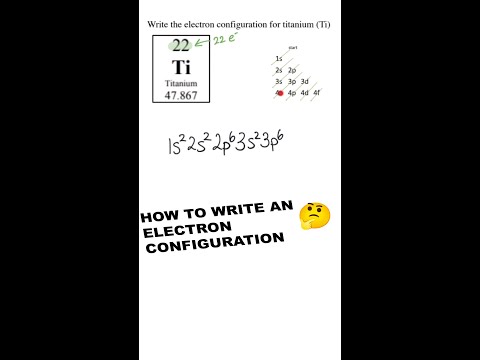 0:01:00
0:01:00
 0:03:25
0:03:25
 0:13:36
0:13:36
 0:00:48
0:00:48
 0:03:20
0:03:20
 0:00:16
0:00:16