filmov
tv
Mass spectrometry for proteomics - part 2

Показать описание
Intro to Proteomics / Mass Spectrometry (MS)
MS-based proteomics: A short introduction to the core concepts of proteomics and mass spectrometry
Mass spectrometry for proteomics - part one
Mass spectrometry for proteomics - part 2
Proteomics 101
Mass Spectrometry-Based Proteomics | 2021 EMSL Summer School
Mass Spectrometry in Proteomics
Introduction into data analysis for mass spectrometry-based proteomics - Lecture by Lennart Martens
Mass Spectrometry: Amanda Paulovich, Principles of Proteomics Series
023-Mass Spectroscopy
Mass Spectrometry
Introduction to Proteomics | 2021 EMSL Summer School
Mass spectrometry
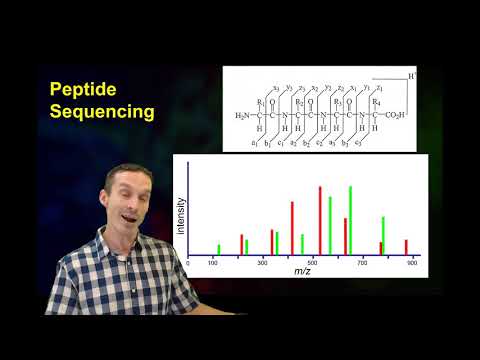
BroadE: Fundamentals of peptide and protein mass spectrometry
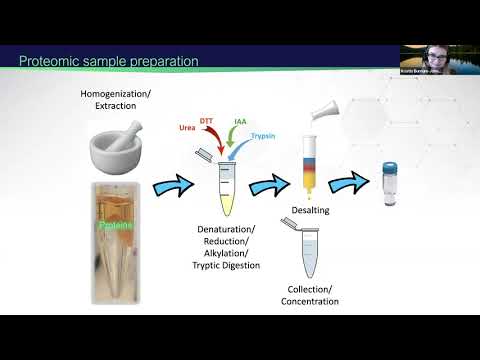
⚖️ How to PREPARE SAMPLES FOR MASS SPECTROMETRY || Proteomics || Protein Analysis Via Mass Spec
Quickly Understand Tandem Mass Spectrometry (MS/MS)
NNFC Workshop: Lili Niu, Mass-spectrometry-based Proteomics for biomarker discovery and pQTL mapping
Mass spectrometry | Atomic structure and properties | AP Chemistry | Khan Academy
Top down vs bottom up proteomics
R12. Mass Spectrometry of the Cysteine Proteome
CMFI Mass Spec Seminar #16 - Proteomics MS/MS Data Aquistion
MALDI-TOF MS | What are the applications of MALDI-TOF? | Mass spectrometry.
Mayo Clinic Cancer Center Proteomics Core: A Virtual Tour
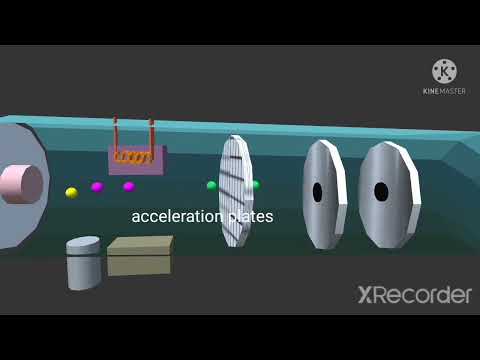
Mass spectrometer animation
Комментарии
 0:21:07
0:21:07
 0:10:59
0:10:59
 0:23:00
0:23:00
 0:09:58
0:09:58
 0:02:33
0:02:33
 0:43:55
0:43:55
 0:05:53
0:05:53
 2:50:52
2:50:52
 0:01:23
0:01:23
 0:04:43
0:04:43
 0:04:51
0:04:51
 0:43:34
0:43:34
 0:08:47
0:08:47
 0:49:56
0:49:56
 0:15:38
0:15:38
 0:03:27
0:03:27
 0:36:27
0:36:27
 0:04:18
0:04:18
 0:17:12
0:17:12
 0:53:50
0:53:50
 1:01:39
1:01:39
 0:05:49
0:05:49
 0:02:11
0:02:11
 0:01:18
0:01:18