filmov
tv
Mass spectrometry
Показать описание
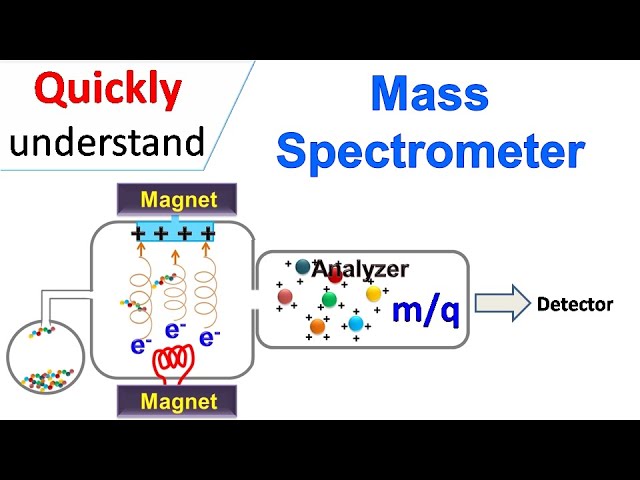
Mass spectrometry (Mass spectrometer) is the analytical technique that measures the mass to charge ratio of ions. In this technique the sample molecules are converted into ions by several methods such as: electron impact ionisation, Chemical ionisation, or electrospray ionisation. Once the ions are generated they are allowed to enter the mass analyser where they are separated by magnetic field based on their mass to charge ratio.
Mass spectrometry | Atomic structure and properties | AP Chemistry | Khan Academy
Mass Spectrometry
Mass Spectrometry
2.2 The Mass Spectrometer
How the accelerator mass spectrometer works – Ian Clark, University of Ottawa
Mass spectrometer animation
Mass Spectrometry - Interpretation Made Easy!
Mass Spectrometry
IR Spectroscopy and Mass Spectrometry: Crash Course Organic Chemistry #5
Mass spectrometry
Lecture 4. Mass Spectrometry: Theory, Instrumentation, and Techniques
How it Works: Mass Spectrometer
Spectrometry | Chemical Tests | Chemistry | FuseSchool
14.4 Introduction to Mass Spectrometry | Organic Chemistry
The Main Purpose of Mass Spectrometry #organicchemistry
Mass Spectrometry Tutorial: How to Tune Your Analytes
What is a mass spec instrument? Interview with Mike Rosenblatt
DIY mass spectrometer measures potassium in dietary salt substitute
An Animated Lesson on Mass Spectrometry
Mass Spectrometry & Fragmentation - A-level Chemistry
Thermo Scientific Orbitrap Exploris GC Mass Spectrometer
Mass Spectrometry
HOW TO INTERPRET MASS SPECTROMETRY GRAPHS
Mass Spectrometry | Principle and Instrumentation | Velocity Selector
Комментарии
 0:04:18
0:04:18
 0:04:51
0:04:51
 0:10:02
0:10:02
 0:02:44
0:02:44
 0:04:12
0:04:12
 0:01:18
0:01:18
 0:13:07
0:13:07
 0:08:20
0:08:20
 0:13:51
0:13:51
 0:08:47
0:08:47
 0:55:22
0:55:22
 0:02:59
0:02:59
 0:03:51
0:03:51
 0:06:19
0:06:19
 0:00:59
0:00:59
 0:17:02
0:17:02
 0:00:44
0:00:44
 0:26:45
0:26:45
 0:19:33
0:19:33
 0:10:46
0:10:46
 0:00:31
0:00:31
 0:09:37
0:09:37
 0:07:41
0:07:41
 0:13:59
0:13:59