filmov
tv
Internal Energy Total - Heat and WorkPV 001

Показать описание
As a system increases in volume, it absorbs 52.5 J of energy in the form of heat from the surroundings. The piston is working against a pressure of 0.550 atm. The final volume of the system is 58.0 L. What was the initial volume of the system if the internal energy of the system decreased by 103.4 J?
Internal Energy
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
The First Law of Thermodynamics: Internal Energy, Heat, and Work
Internal Energy Total - Heat and WorkPV 001
GCSE Physics - Internal Energy and Specific Heat Capacity
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems
Heat Capacity, Specific Heat, and Calorimetry
Internal Energy Definition - A Level Physics
INTERNAL ENERGY | INTRINSIC ENERGY | THERMODYNAMICS | INTERNAL ENERGY IN THERMODYNAMICS |
Internal energy change
ENTHALPY and INTERNAL ENERGY in 12 Minutes!
Internal Energy of an Ideal Gas - Molar Heat Capacity of Monatomic & Diatomic Gases, Gamma Ratio...
Understanding Internal Energy | Class 11 Physics | Thermodynamics Explained
Thermodynamics 1- Heat, Work and Internal Energy
Heat, Internal Energy, and Enthalpy
Calculate Total Change In Internal Energy (∆E) Using Heat and WorkPV 002
Internal Energy and Heat Capacity and Equipartition 4449 L1-5 2020
First Law of Thermodynamics.
Thermodynamics - Heat, Work & Internal Energy: Internal Energy | Aakash Institute
Calculate Total Internal Energy Change (∆E) From Heat and WorkPV 003
What Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity
Ideal Gases - Specific Heat, Internal Energy, Enthalpy | Thermodynamics | (Solved Problems)
Temperature, Thermal Energy, and Heat - IB Physics
What is Thermodynamics? | Class 11 Physics Explained
Комментарии
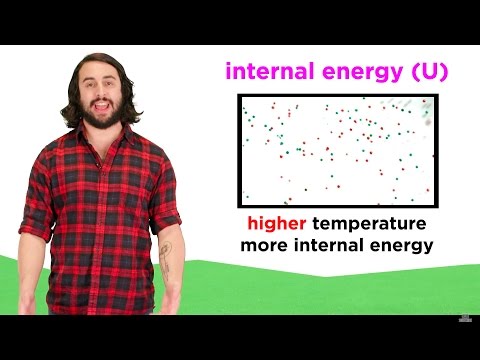 0:03:48
0:03:48
 0:11:27
0:11:27
 0:05:44
0:05:44
 0:08:26
0:08:26
 0:04:36
0:04:36
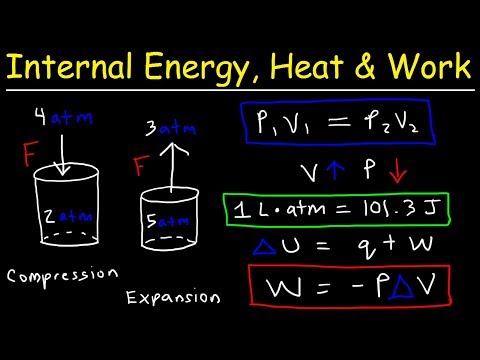 0:23:29
0:23:29
 0:04:14
0:04:14
 0:00:09
0:00:09
 0:00:14
0:00:14
 0:01:55
0:01:55
 0:11:52
0:11:52
 0:10:36
0:10:36
 0:00:44
0:00:44
 0:19:27
0:19:27
 0:05:31
0:05:31
 0:05:36
0:05:36
 0:27:46
0:27:46
 0:00:29
0:00:29
 0:04:47
0:04:47
 0:10:08
0:10:08
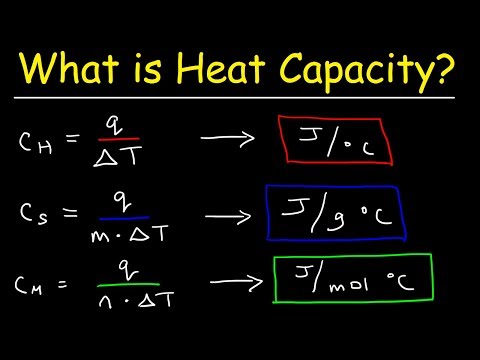 0:12:29
0:12:29
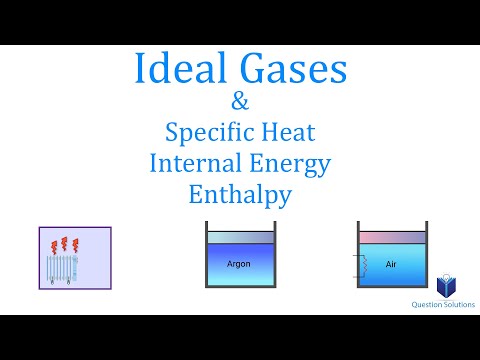 0:11:25
0:11:25
 0:11:23
0:11:23
 0:00:53
0:00:53