filmov
tv
Using Calorimetry to Calculate Enthalpies of Reaction - Chemistry Tutorial
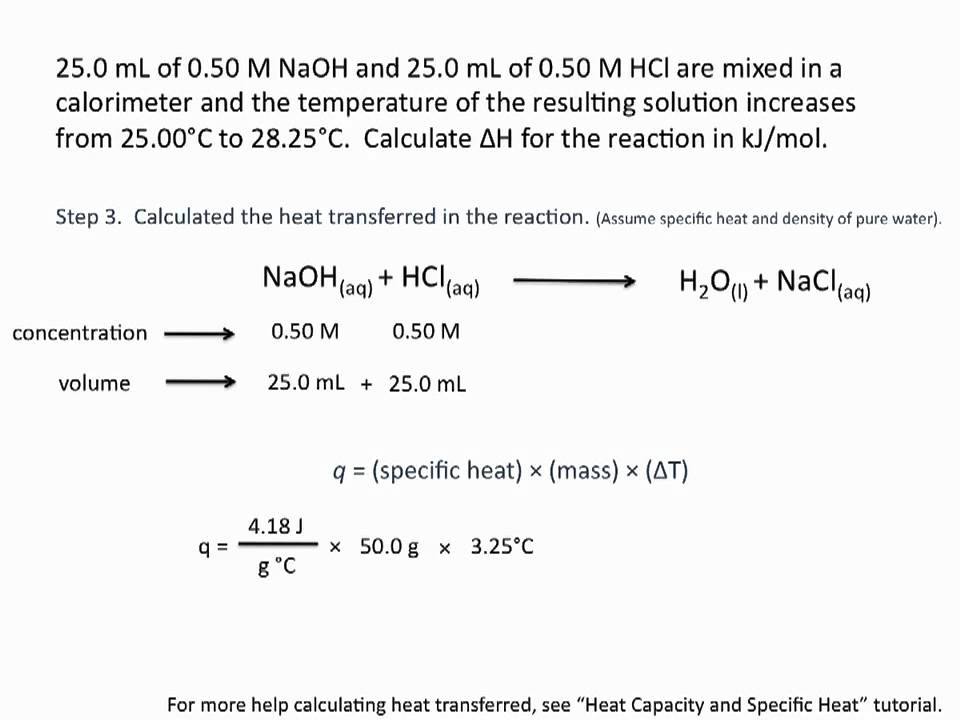
Показать описание
This tutorial covers how to calculate enthalpies of reactions using calorimetry and includes examples of how to calculate the heat released or absorbed by a particular amount of material, and how to use this information and the specific amount of material used to calculate the enthalpy of the reaction in the standard units of kJ/mol.
Using Calorimetry to Calculate Enthalpies of Reaction - Chemistry Tutorial
Calorimetry: Calculate Enthalpy
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Tutorial
Coffee Cup Calorimeter - Calculate Enthalpy Change, Constant Pressure Calorimetry
20.5.3 - Calculating Enthalpy Using Calorimetry
Using Calorimetry to determine molar enthalpy
Sample Calculation - Calculating Enthalpy of Reaction from Calorimetry
Calculating enthalpy changes and calorimetry (Chemical Energetics #2)
Bomb Calorimeter vs Coffee Cup Calorimeter Problem - Constant Pressure vs Constant Volume Calorimet
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry
6 Calorimetry Calculations (neutralisation)
Required practical 2: Measurement of an enthalpy change
Calculating enthalpy change
Hess's Law Problems & Enthalpy Change - Chemistry
Experiment 2 - Determination of Enthalpy Change by Calorimetry
Calorimetry Lab Calculations explained
Calorimetry: How to calculate enthalpy change experimentally
Calorimetry Problems: Finding the Heat of Reaction Using Calorimetry
5.1 Calculating enthalpy changes (SL)
Section 2-Enthalpy and Calorimetry
R1.1.4 Calculating ΔH using q = mcΔT
Energetics 2 | Calorimetry | Enthalpy Change Experiments | A level
ENERGY UNIT: Calculating enthalpy (heat absorption or release) using calorimetry
Heat Capacity, Specific Heat, and Calorimetry
Комментарии
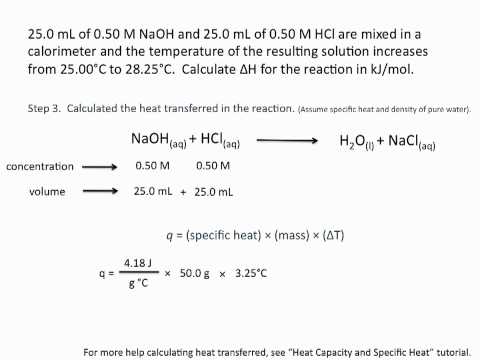 0:09:06
0:09:06
 0:09:57
0:09:57
 0:09:06
0:09:06
 0:10:40
0:10:40
 0:09:26
0:09:26
 0:04:57
0:04:57
 0:10:46
0:10:46
 0:19:49
0:19:49
 0:10:11
0:10:11
 0:05:03
0:05:03
 0:08:12
0:08:12
 0:07:09
0:07:09
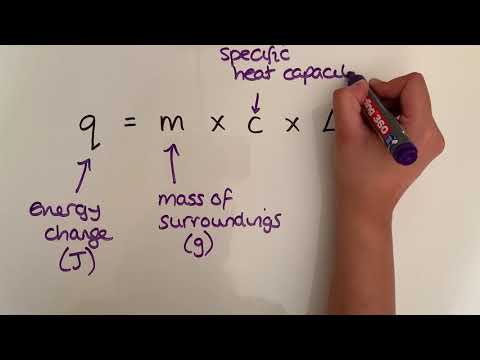 0:07:22
0:07:22
 0:14:03
0:14:03
 0:09:00
0:09:00
 0:08:15
0:08:15
 0:11:02
0:11:02
 0:05:30
0:05:30
 0:08:01
0:08:01
 0:14:52
0:14:52
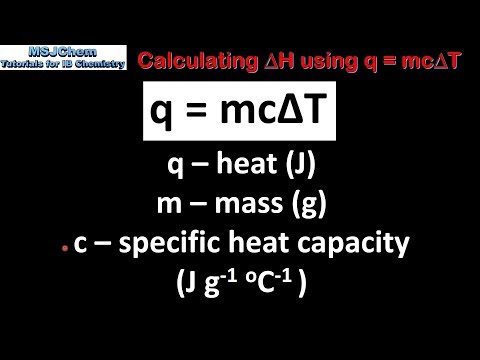 0:03:35
0:03:35
 0:56:24
0:56:24
 0:09:45
0:09:45
 0:04:14
0:04:14