filmov
tv
Calculating enthalpy changes and calorimetry (Chemical Energetics #2)

Показать описание
LESSON OBJECTIVE: Understand how to calculate the enthalpy change of a reaction from experimental data obtained via calorimetry.
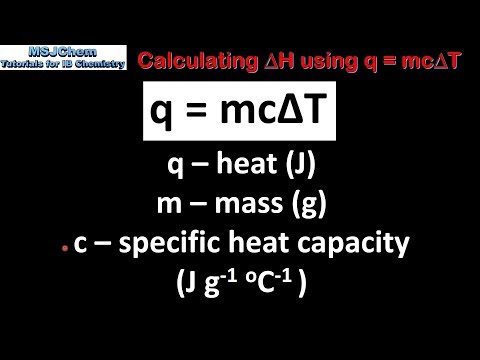
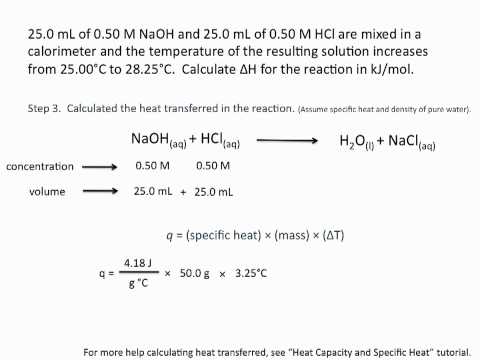
In this lesson we discuss how to calculate enthalpy changes using the equation ΔH=–mcΔT, specific heat capacity and the concept of calorimetry as an experimental technique. This is lesson sixteen in our physical chemistry series for Unit 5: Chemical Energetics (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
Download the slides and student led tasks for this lesson here:
LEARNING OUTCOMES (from the Cambridge International AS and A Level Chemistry (9701) 2019-2021 curriculum):
5.1 Enthalpy change, ΔH
c) calculate enthalpy changes from appropriate experimental results, including the use of the relationship ΔH = –mcΔT
 0:09:57
0:09:57
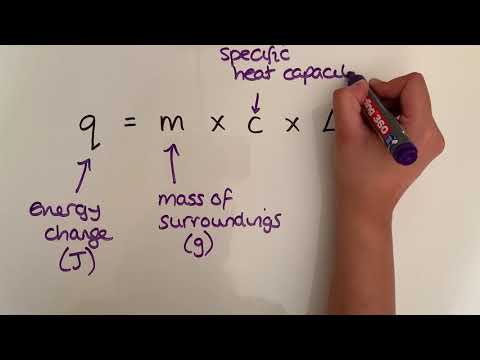 0:07:22
0:07:22
 0:10:40
0:10:40
 0:07:09
0:07:09
 0:19:49
0:19:49
 0:04:14
0:04:14
 0:14:03
0:14:03
 0:11:56
0:11:56
 0:09:00
0:09:00
 0:08:01
0:08:01
 0:56:24
0:56:24
 0:03:35
0:03:35
 0:27:03
0:27:03
 0:05:03
0:05:03
 0:05:50
0:05:50
 0:09:06
0:09:06
 0:26:51
0:26:51
 0:15:26
0:15:26
 0:17:47
0:17:47
 0:08:12
0:08:12
 0:10:38
0:10:38
 0:11:06
0:11:06
 0:11:02
0:11:02
 0:04:13
0:04:13