filmov
tv
Law of Conservation of Mass - Fundamental Chemical Laws, Chemistry
Показать описание
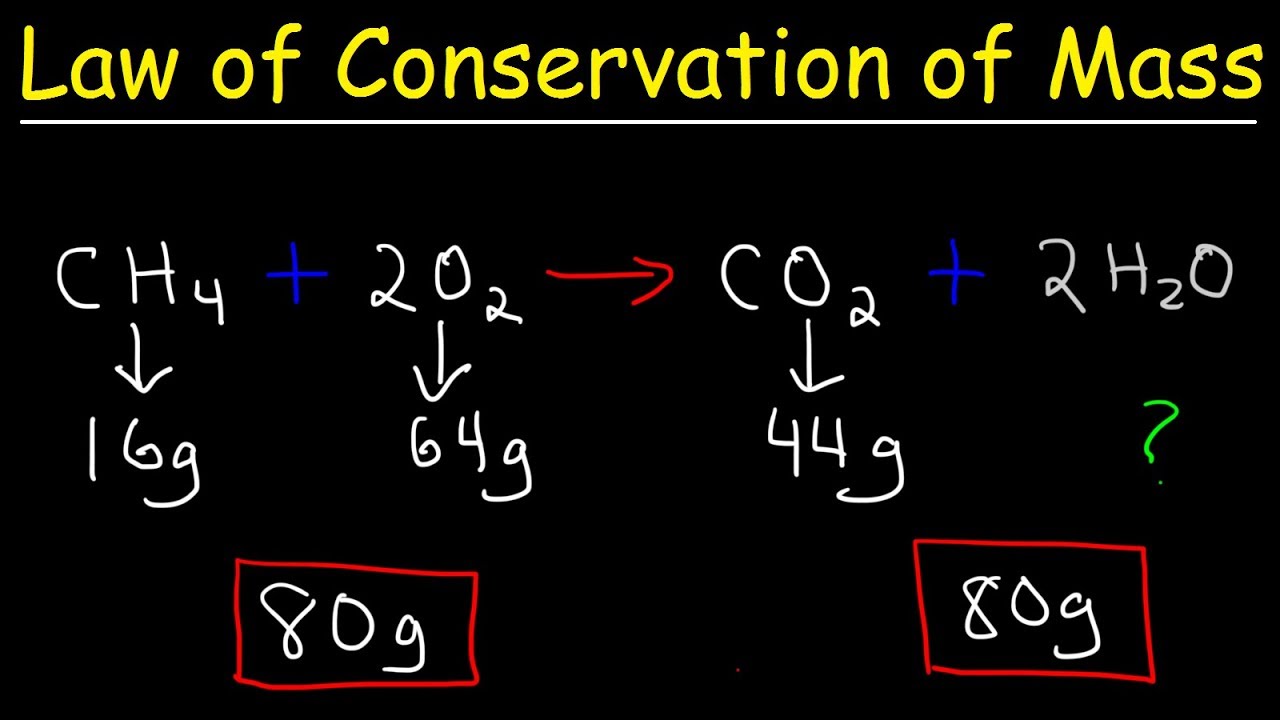
This chemistry video tutorial discusses the law of conservation of mass and provides examples associated with chemical reactions. The conservation of mass in a chemical reaction is one of the fundamental laws in chemistry. The total mass of the reactants must equal the total mass of the products in a chemical reaction.
Chemistry - Basic Introduction:
Scientific Notation Review:
Significant Figures Review:
Unit Conversion Problems:
Accuracy and Precision:
Density Practice Problems:
________________________________
Pure Substances & Mixtures:
Homogeneous & Heterogeneous Mixtures:
Physical and Chemical Changes:
Solids, Liquids, Gases, & Plasma:
Physical Vs Chemical Properties:
__________________________________
Law of Conservation of Mass:
Law of Definite Proportions:
Law of Multiple Proportions:
Rutherford's Gold Foil Experiment:
Cathode Ray Tube Experiment:
_________________________________
Atoms - Basic Introduction:
Cations and Anions Explained:
Diatomic Elements & Molecules:
Elements, Atoms, & Molecules:
Protons, Neutrons, & Electrons:
_______________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry - Basic Introduction:
Scientific Notation Review:
Significant Figures Review:
Unit Conversion Problems:
Accuracy and Precision:
Density Practice Problems:
________________________________
Pure Substances & Mixtures:
Homogeneous & Heterogeneous Mixtures:
Physical and Chemical Changes:
Solids, Liquids, Gases, & Plasma:
Physical Vs Chemical Properties:
__________________________________
Law of Conservation of Mass:
Law of Definite Proportions:
Law of Multiple Proportions:
Rutherford's Gold Foil Experiment:
Cathode Ray Tube Experiment:
_________________________________
Atoms - Basic Introduction:
Cations and Anions Explained:
Diatomic Elements & Molecules:
Elements, Atoms, & Molecules:
Protons, Neutrons, & Electrons:
_______________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:03:14
0:03:14
 0:04:37
0:04:37
 0:04:04
0:04:04
 0:03:05
0:03:05
 0:03:48
0:03:48
 0:05:53
0:05:53
 0:01:39
0:01:39
 0:03:00
0:03:00
 0:23:40
0:23:40
 0:03:30
0:03:30
 0:02:18
0:02:18
 0:01:25
0:01:25
 0:02:33
0:02:33
 0:10:59
0:10:59
 0:04:11
0:04:11
 0:01:58
0:01:58
 0:02:39
0:02:39
 0:10:41
0:10:41
 0:01:00
0:01:00
 0:06:21
0:06:21
 0:02:07
0:02:07
 0:03:51
0:03:51
 0:03:44
0:03:44
 0:02:27
0:02:27