filmov
tv
Bent Molecular Geometry/Shape and Bond Angles

Показать описание
In this video we’ll look at the Bent Molecular Geometry and Bond Angles. We'll use the example of H2O to understand the molecular shape. To do that we'll use VSEPR Theory and the Lewis Structure for H2O and then use interactive models and visualization to visualize the Bent geometry.
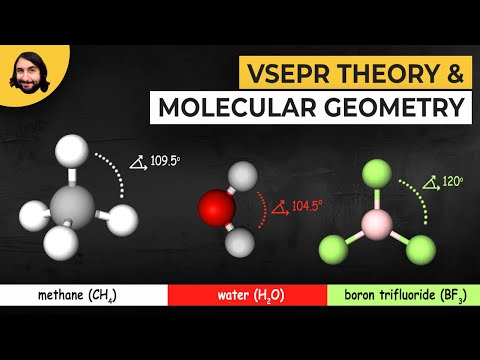
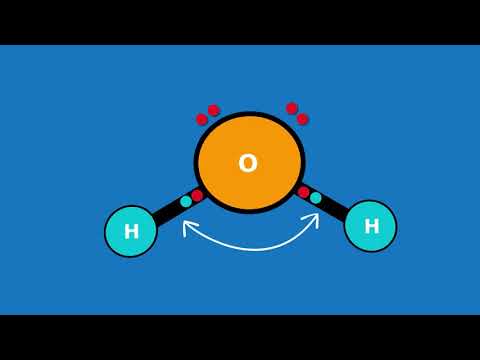
The Bent molecular shape occurs when there are two atoms attached to the central atom and two lone pairs (unbonded pairs) of electrons on the central atom. H2O is a good example of a Bent molecular geometry.
It is useful to understand the generic molecular geometry and then apply it to specific molecules using either the steric number and number of lone pairs of electrons or the AXE notation.
For the more on the molecular geometries below see my video at:
- Linear
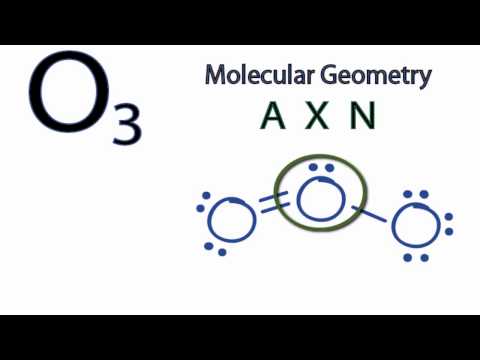
- Bent (90 and 120-degree bond angles)
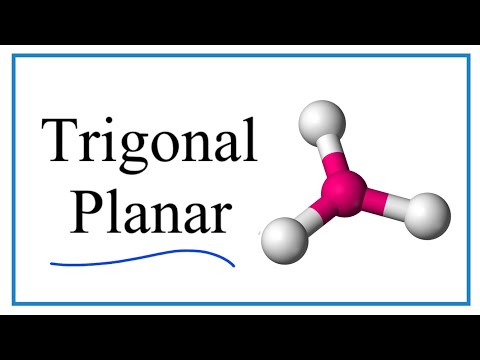
- Trigonal Planer
- Trigonal Pyramidal
- Tetrahedral
- Trigonal Bipyramidal
- Octahedral
The role of lone pairs (unbonded electron pairs) and their VSEPR influence on molecule shape will also be addressed in the video.
Molecular Shapes done with PhET's free online website:
More info on the Bent molecular geometry at:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
The Bent molecular shape occurs when there are two atoms attached to the central atom and two lone pairs (unbonded pairs) of electrons on the central atom. H2O is a good example of a Bent molecular geometry.
It is useful to understand the generic molecular geometry and then apply it to specific molecules using either the steric number and number of lone pairs of electrons or the AXE notation.
For the more on the molecular geometries below see my video at:
- Linear
- Bent (90 and 120-degree bond angles)
- Trigonal Planer
- Trigonal Pyramidal
- Tetrahedral
- Trigonal Bipyramidal
- Octahedral
The role of lone pairs (unbonded electron pairs) and their VSEPR influence on molecule shape will also be addressed in the video.
Molecular Shapes done with PhET's free online website:
More info on the Bent molecular geometry at:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Комментарии
 0:03:25
0:03:25
 0:02:20
0:02:20
 0:06:31
0:06:31
 0:13:10
0:13:10
 0:11:01
0:11:01
 0:01:45
0:01:45
 0:01:48
0:01:48
 0:01:37
0:01:37
 0:01:50
0:01:50
 0:03:03
0:03:03
 0:00:06
0:00:06
 0:01:37
0:01:37
 0:01:35
0:01:35
 0:01:46
0:01:46
 0:02:07
0:02:07
 0:02:05
0:02:05
 0:05:28
0:05:28
 0:01:50
0:01:50
 0:13:23
0:13:23
 0:09:36
0:09:36
 0:01:30
0:01:30
 0:01:52
0:01:52
 0:05:38
0:05:38
 0:06:35
0:06:35