filmov
tv
C2H2 Molecular Geometry / Shape and Bond Angles (see description for note)

Показать описание
A quick explanation of the molecular geometry of C2H2 including a description of the C2H2 bond angles. Note, the Hydrogen atoms (H) should not have lone pair electrons!
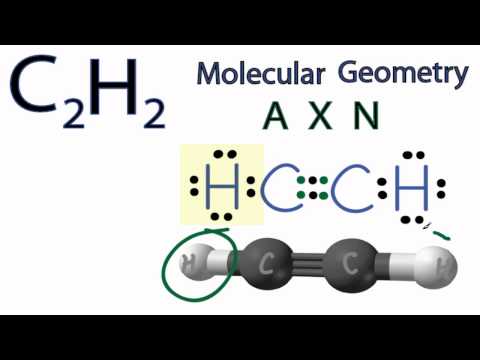
We'll determine the C2H2 molecular geometry with respect to the Carbon on the right (the other Carbon atom will have the same shape since they are symmetrical).
Looking at the C2H2 Lewis structure we can see that there are two atoms attached to the Carbon of interest and that there no lone pair electrons. Based on VSEPR Theory (Valence Shell Electron Pair Repulsion Theory) the electron clouds on the atoms around the C will repel each other. As a result they will be pushed apart giving the molecule a linear molecular geometry or shape.
The C2H2 bond angle will be 180 degrees since it has a linear geometry.
Helpful Resources:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
We'll determine the C2H2 molecular geometry with respect to the Carbon on the right (the other Carbon atom will have the same shape since they are symmetrical).
Looking at the C2H2 Lewis structure we can see that there are two atoms attached to the Carbon of interest and that there no lone pair electrons. Based on VSEPR Theory (Valence Shell Electron Pair Repulsion Theory) the electron clouds on the atoms around the C will repel each other. As a result they will be pushed apart giving the molecule a linear molecular geometry or shape.
The C2H2 bond angle will be 180 degrees since it has a linear geometry.
Helpful Resources:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
C2H2 Molecular Geometry, Shape and Bond Angles(Acetylene)
C2H2 Molecular Geometry / Shape and Bond Angles (see description for note)
How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)
Draw the Orbital Overlap Diagram of C2H2 (ethyne, acetylene)
3D Molecular Structure of Ethylene (Ethene) #ethylene #chemistry
Acetylene or Ethyne: 3D Molecule
VSEPR Theory and Molecular Geometry
Orbital Overlap Diagram of Ethyne - Acetylene
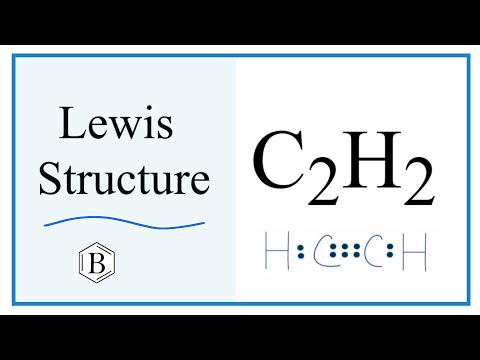
C2H2 Lewis Structure (Ethyne or Acetylene)
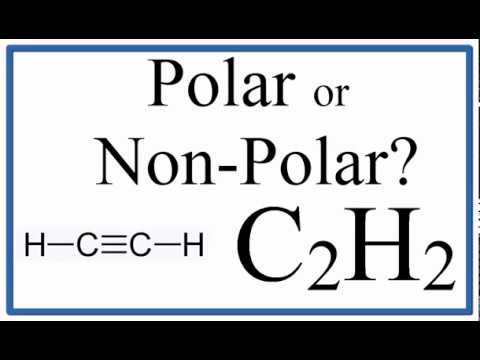
Is C2H2 Polar or Non-polar? (Ethyne or Acetylene)
Hybridization of C2H2 (ethyne)
How To Draw Lewis Structures
Linear Molecular Geometry/Shape and Bond Angles
How do you draw the Lewis structure for C2H2? ||Ethyne or Acetylene Lewis Structure
VSEPR Theory - Acetylene (C2H2)
Chemical bonding IIT Questions No 2 (IX Class)
Hybridisation of C2H2 || sp hybridisation || Formation of Ethyne molecule
H2C2 Lewis Structure (Ethyne or Acetylene)
C2H4 Molecular Geometry / Shape and Bond Angles
How to draw Lewis structure of any compound? Easy Trick
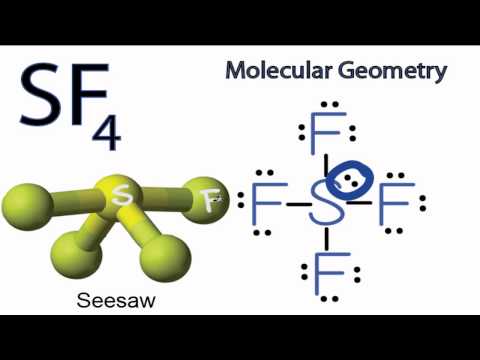
SF4 Molecular Geometry / Shape
How To Make 3D MOLECULAR MODELS Using STYRO BALL | IN CHEMISTRY For Your School Project |
electron dot structure of ethyne C2H2 Lewis structure of ethyne C2H2 carbon and it's compounds
Water Molecular Geometry and Bond Angles
Комментарии
 0:01:57
0:01:57
 0:01:30
0:01:30
 0:02:25
0:02:25
 0:10:06
0:10:06
 0:00:44
0:00:44
 0:00:32
0:00:32
 0:06:31
0:06:31
 0:06:20
0:06:20
 0:02:51
0:02:51
 0:01:18
0:01:18
 0:03:26
0:03:26
 0:11:50
0:11:50
 0:01:37
0:01:37
 0:04:42
0:04:42
 0:03:20
0:03:20
 0:00:51
0:00:51
 0:05:31
0:05:31
 0:01:16
0:01:16
 0:01:52
0:01:52
 0:06:19
0:06:19
 0:00:57
0:00:57
 0:09:17
0:09:17
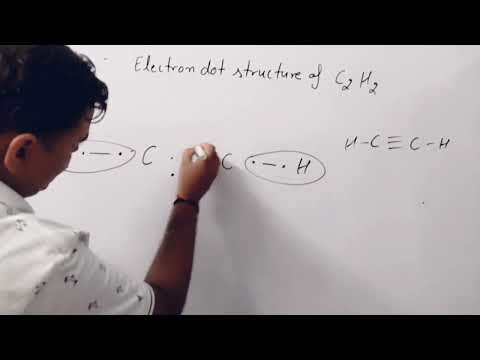 0:01:27
0:01:27
 0:02:23
0:02:23