filmov
tv
Beer's Law Overview

Показать описание
Beer's Law Overview
Spectrophotometry and Beer's Law
Spectroscopy || Beer- Lambert's Law
Beer Lambert's Law, Absorbance & Transmittance - Spectrophotometry, Basic Introduction - Ch...
Beers Law Introduction
Introduction to beer law
Spectrophotometry and the Beer–Lambert Law | AP Chemistry | Khan Academy
Beer's Law
AP Chemistry Lab: Beer's Law
Introduction to Beer Law
Beer's Law - 5 minute review summary!
Beer Lambert Law
Beers Law PhET Simulation introduction
Beer's law #chemistry #bloodtest #medtechstudent #cls #mls #laboratory #concentration #absorpti...
Beer's Law
Beer-Lambert Law in Spectroscopy #organicchemistry
Beer's Law - What is Beer's Law and How to do Problems relating to it!
3-6 Beers Law
Worked example: Calculating concentration using the Beer–Lambert law | AP Chemistry | Khan Academy
Beers Law
Beer's Law
The Beer - Lambert Law
Beer's Law and It's Application
Spectrophotometry: Learn the Beer-Lambert law with absorbance experiments | Virtual Lab
Комментарии
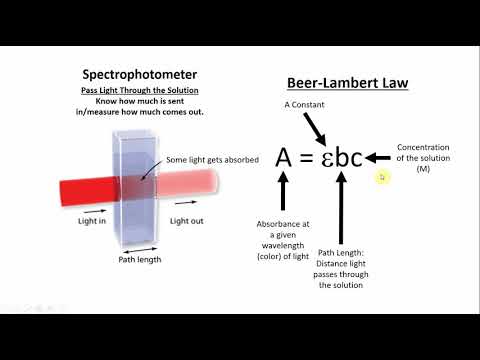 0:08:37
0:08:37
 0:06:25
0:06:25
 0:06:38
0:06:38
 0:18:13
0:18:13
 0:07:03
0:07:03
 0:05:17
0:05:17
 0:10:23
0:10:23
 0:01:33
0:01:33
 0:01:12
0:01:12
 0:19:55
0:19:55
 0:06:28
0:06:28
 0:00:33
0:00:33
 0:01:50
0:01:50
 0:00:15
0:00:15
 0:04:39
0:04:39
 0:00:33
0:00:33
 0:16:40
0:16:40
 0:05:11
0:05:11
 0:03:48
0:03:48
 0:23:04
0:23:04
 0:00:22
0:00:22
 0:05:53
0:05:53
 0:04:43
0:04:43
 0:00:51
0:00:51