filmov
tv
Beers Law

Показать описание
Introduction to Beer's Law - analyzing data graphically with colorimetry to determine the concentration of an unknown coloured solution
Spectrophotometry and Beer's Law
Beer Lambert's Law, Absorbance & Transmittance - Spectrophotometry, Basic Introduction - Ch...
Spectroscopy || Beer- Lambert's Law
Beer's Law Overview
Spectrophotometry and the Beer–Lambert Law | AP Chemistry | Khan Academy
Beer's Law and It's Application
Investigate Beer’s Law with Go Direct® SpectroVis Plus Spectrophotometer
Beer's Law Experiment - Calibration Curve With Nickel (II) Sulfate Vernier LabQuest2
Derivation of Beer Lambert Law
Beer's Law
Spectrophotometers, calibration curves and Beer's Law
Beer's Law #shorts
Basic Concept of Lambert Beer's Law || Lambert Beer's Law
Beer's Law Simulation Tutorial
Beers Law
UV interview question answers | UV principle | Beer-Lambert's law #uv #chemistry
PASCO Spectrometer: Beer's Law
Beer's law #chemistry #bloodtest #medtechstudent #cls #mls #laboratory #concentration #absorpti...
Virtual Simulation for Beer Lambert Law | Science Experiment
Beer-Lambert Law in Spectroscopy #organicchemistry
Beer's Law Photochemistry #chemistry #photochemistry Beer Lambert law #spectroscopy #bsc #msc #...
Spectrophotometry and the Beer-Lambert Law - AP Chem Unit 3, Topic 13
Beer Lambert Law simplest explanation| animation
Beer's Law: Concentrate on Concentration
Комментарии
 0:06:25
0:06:25
 0:18:13
0:18:13
 0:06:38
0:06:38
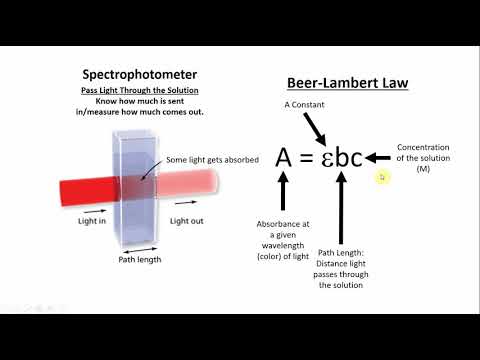 0:08:37
0:08:37
 0:10:23
0:10:23
 0:04:43
0:04:43
 0:06:46
0:06:46
 0:08:20
0:08:20
 0:04:30
0:04:30
 0:08:16
0:08:16
 0:11:58
0:11:58
 0:00:58
0:00:58
 0:11:21
0:11:21
 0:02:30
0:02:30
 0:23:04
0:23:04
 0:00:26
0:00:26
 0:06:35
0:06:35
 0:00:15
0:00:15
 0:05:29
0:05:29
 0:00:33
0:00:33
 0:00:06
0:00:06
 0:20:23
0:20:23
 0:07:09
0:07:09
 0:01:02
0:01:02