filmov
tv
Beer-Lambert Law in Spectroscopy #organicchemistry

Показать описание
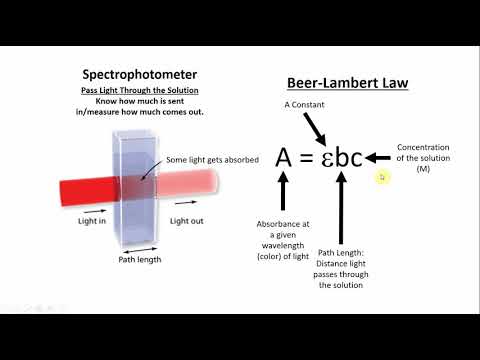
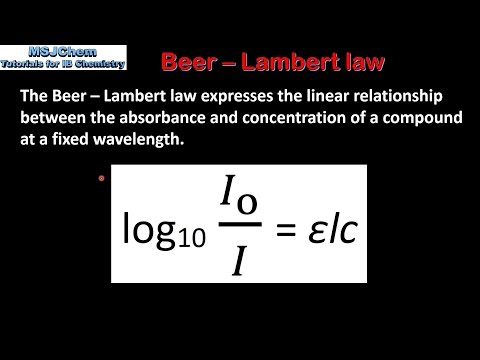
The Beer-Lambert Law states that the amount of energy absorbed by a solution is proportional to the path length and concentration. Put simply, a more concentrated solution absorbs more light than a dilute solution does.
The Beer-Lambert Law equation is as follows: A = ϵ.d.c
Where ϵ = molar absorptivity, d = path length and c = concentration.
Molar absorptivity is a unique physical constant of the sample that relates to the sample’s ability to absorb light at a given wavelength. ϵ has the unit as L·mol-1·cm-1.
#chemistry #mettlertoledo #ftir #spectroscopy #fouriertransform #atr #science #chemicalengineering #education #beerslaw #beerlambertlaw #absorbance #chemicalreactionengineering
The Beer-Lambert Law equation is as follows: A = ϵ.d.c
Where ϵ = molar absorptivity, d = path length and c = concentration.
Molar absorptivity is a unique physical constant of the sample that relates to the sample’s ability to absorb light at a given wavelength. ϵ has the unit as L·mol-1·cm-1.
#chemistry #mettlertoledo #ftir #spectroscopy #fouriertransform #atr #science #chemicalengineering #education #beerslaw #beerlambertlaw #absorbance #chemicalreactionengineering
 0:06:25
0:06:25
 0:18:13
0:18:13
 0:06:38
0:06:38
 0:00:33
0:00:33
 0:00:33
0:00:33
 0:10:23
0:10:23
 0:08:37
0:08:37
 0:07:35
0:07:35
 0:02:29
0:02:29
 0:00:06
0:00:06
 0:00:05
0:00:05
 0:05:53
0:05:53
 0:00:07
0:00:07
 0:02:09
0:02:09
 0:00:08
0:00:08
 0:11:21
0:11:21
 0:07:44
0:07:44
 0:00:31
0:00:31
 0:04:02
0:04:02
 0:00:06
0:00:06
 0:06:16
0:06:16
 0:03:00
0:03:00
 0:12:11
0:12:11
 0:00:15
0:00:15