filmov
tv
Spectroscopy || Beer- Lambert's Law

Показать описание
#biologyanimation #biophysics #spectroscopy #spectrophotometer
Today we are going to learn about the spectroscopy and beer lambert’s law.
What is spectroscopy?
It is the study of the interaction between electromagnetic radiation and matter.
This matter can be any atoms, molecules or ions.
The electromagnetic spectrum ranges from very short wavelengths like gamma rays to long wavelengths such as radio waves.
The visible range is approx. 400-700 nanometer.
Now, coming to the types of spectroscopy.
When electromagnetic radiation meets the matter, the matter may absorb emit or scatter the radiation.
Depend on which there are three types of spectroscopy-absorption, emission and scattering.
Today we will talk about only absorption spectroscopy.
The basic principle of absorption spectroscopy is when a beam of monochromatic light passes through a solution or matter, some of its radiation may be absorbed.
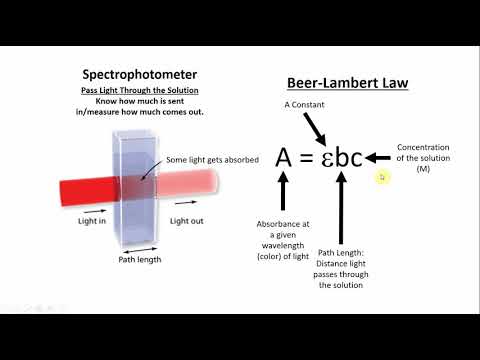
This absorbance is measured with the help of an instrument known as a spectrophotometer.
Beer-Lambert’s law is based on two different laws.
The lambert’s law states that, when a monochromatic light passes through a transparent medium, the intensity of transmitted light decreases exponentially as the thickness of absorbing material increases.
Beer’s law states that the intensity of transmitted monochromatic light decreases exponentially as the concentration of the absorbing substance increases.
This Beer-Lambert’s law can be expressed mathematically by the formula
A=log I_o/I=εcl
Where A refers to the absorbance, I_o & I is the intensity of incident & transmitted light, respectively.
ε is the excitation coefficient which is constant for a particular matter.
C is the concentration of the sample & l is the path length.
T refers to the transmittance which is expressed by the ratio of I and I_o.
If we derive the relationship of absorbance with the percent transmittance from the above formula, we get absorbance is equal to 2-〖log〗_10%T
So, in spectrophotometer, you can get both the reading of absorbance & transmittance.
Now, see if we increase the sample concentration, the absorbance increases while the transmittance decreases.
However, if we change the path length, the absorbance increases and the transmittance decreases further.
Now, coming to the applications of the spectrophotometer, for example, we are taking protein estimation.
Let you have a sample of unknown protein concentration with you and you want to estimate it’s concentration.
First, you have to take a series of known concentrations of protein along with a blank.
Now you will add reagents to all the tubes.
The protein will give color after reacting with reagents.
The color intensity is directly proportional to the protein concentrations.
Now you have to measure the absorbance for each known concentration of the proteins along with your sample.
When you plot those readings in a graph, it will give you a standard curve.
From the standard curve, the value of 1 absorbance is calculated.
Now using the formula, you can calculate the protein concentration of the unknown sample.
Apart from protein estimation, spectroscopy is used to measure DNA/RNA concentration in enzymatic assays, in ELISA or measuring any concentrations of molecules which give any color of the UV-Visible range when in a solution.
Today we are going to learn about the spectroscopy and beer lambert’s law.
What is spectroscopy?
It is the study of the interaction between electromagnetic radiation and matter.
This matter can be any atoms, molecules or ions.
The electromagnetic spectrum ranges from very short wavelengths like gamma rays to long wavelengths such as radio waves.
The visible range is approx. 400-700 nanometer.
Now, coming to the types of spectroscopy.
When electromagnetic radiation meets the matter, the matter may absorb emit or scatter the radiation.
Depend on which there are three types of spectroscopy-absorption, emission and scattering.
Today we will talk about only absorption spectroscopy.
The basic principle of absorption spectroscopy is when a beam of monochromatic light passes through a solution or matter, some of its radiation may be absorbed.
This absorbance is measured with the help of an instrument known as a spectrophotometer.
Beer-Lambert’s law is based on two different laws.
The lambert’s law states that, when a monochromatic light passes through a transparent medium, the intensity of transmitted light decreases exponentially as the thickness of absorbing material increases.
Beer’s law states that the intensity of transmitted monochromatic light decreases exponentially as the concentration of the absorbing substance increases.
This Beer-Lambert’s law can be expressed mathematically by the formula
A=log I_o/I=εcl
Where A refers to the absorbance, I_o & I is the intensity of incident & transmitted light, respectively.
ε is the excitation coefficient which is constant for a particular matter.
C is the concentration of the sample & l is the path length.
T refers to the transmittance which is expressed by the ratio of I and I_o.
If we derive the relationship of absorbance with the percent transmittance from the above formula, we get absorbance is equal to 2-〖log〗_10%T
So, in spectrophotometer, you can get both the reading of absorbance & transmittance.
Now, see if we increase the sample concentration, the absorbance increases while the transmittance decreases.
However, if we change the path length, the absorbance increases and the transmittance decreases further.
Now, coming to the applications of the spectrophotometer, for example, we are taking protein estimation.
Let you have a sample of unknown protein concentration with you and you want to estimate it’s concentration.
First, you have to take a series of known concentrations of protein along with a blank.
Now you will add reagents to all the tubes.
The protein will give color after reacting with reagents.
The color intensity is directly proportional to the protein concentrations.
Now you have to measure the absorbance for each known concentration of the proteins along with your sample.
When you plot those readings in a graph, it will give you a standard curve.
From the standard curve, the value of 1 absorbance is calculated.
Now using the formula, you can calculate the protein concentration of the unknown sample.
Apart from protein estimation, spectroscopy is used to measure DNA/RNA concentration in enzymatic assays, in ELISA or measuring any concentrations of molecules which give any color of the UV-Visible range when in a solution.
Комментарии
 0:06:38
0:06:38
 0:06:25
0:06:25
 0:10:23
0:10:23
 0:00:33
0:00:33
 0:18:13
0:18:13
 0:08:37
0:08:37
 0:07:09
0:07:09
 0:00:33
0:00:33
 0:19:23
0:19:23
 0:00:15
0:00:15
 0:00:06
0:00:06
 0:20:23
0:20:23
 0:00:06
0:00:06
 0:04:02
0:04:02
 0:00:51
0:00:51
 0:00:26
0:00:26
 0:08:16
0:08:16
 0:01:23
0:01:23
 0:04:30
0:04:30
 0:02:10
0:02:10
 0:07:44
0:07:44
 0:03:48
0:03:48
 0:12:11
0:12:11
 0:08:02
0:08:02