filmov
tv
Valence Bond Theory

Показать описание
Valence Bond Theory Class 11
For Live Classes, Concept Videos, Quizzes, Mock Tests & Revision Notes please see our Website/App:
Valence Bond Theory (VBT) is a model used in chemistry to describe how atoms combine to form molecules. It provides an explanation for the formation of chemical bonds, emphasizing the role of atomic orbitals and electron pairings. Here are some key aspects of Valence Bond Theory:
1. Atomic Orbitals:
- VBT assumes that atoms use their atomic orbitals to form bonds. Electrons in these orbitals can pair up to form a chemical bond.
- The most commonly involved orbitals are the s, p, and sometimes d orbitals.
2. Hybridization:
- To explain molecular shapes that cannot be accounted for by pure atomic orbitals, VBT introduces the concept of hybridization.
- Hybrid orbitals are formed by mixing different types of atomic orbitals on the same atom (e.g., sp, sp², sp³).
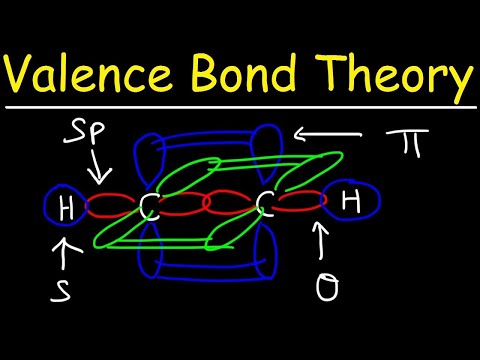
3. Overlap of Atomic Orbitals:
- A chemical bond forms when atomic orbitals overlap, allowing electrons to be shared between atoms.
- The extent of orbital overlap determines the strength of the bond.
4. Sigma (σ) and Pi (π) Bonds:
- Sigma bonds result from the head-to-head overlap of orbitals. They are the first bonds formed between two atoms.
- Pi bonds result from the side-to-side overlap of p orbitals and are found in double and triple bonds, in addition to the sigma bonds.
5. Localized Electron Model:
- VBT treats electrons as localized between two atoms in a bond, unlike Molecular Orbital Theory, which treats electrons as delocalized over the entire molecule.
6. Resonance:
- Some molecules can be represented by more than one valid Lewis structure, known as resonance structures.
- VBT accounts for resonance by considering the molecule as a resonance hybrid of multiple structures.
Valence Bond Theory provides a fundamental framework for understanding chemical bonding in terms of orbital overlap and hybridization. While it has limitations, especially in explaining complex bonding scenarios, it remains a valuable tool for studying the structure and behavior of molecules.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
For Live Classes, Concept Videos, Quizzes, Mock Tests & Revision Notes please see our Website/App:
Valence Bond Theory (VBT) is a model used in chemistry to describe how atoms combine to form molecules. It provides an explanation for the formation of chemical bonds, emphasizing the role of atomic orbitals and electron pairings. Here are some key aspects of Valence Bond Theory:
1. Atomic Orbitals:
- VBT assumes that atoms use their atomic orbitals to form bonds. Electrons in these orbitals can pair up to form a chemical bond.
- The most commonly involved orbitals are the s, p, and sometimes d orbitals.
2. Hybridization:
- To explain molecular shapes that cannot be accounted for by pure atomic orbitals, VBT introduces the concept of hybridization.
- Hybrid orbitals are formed by mixing different types of atomic orbitals on the same atom (e.g., sp, sp², sp³).
3. Overlap of Atomic Orbitals:
- A chemical bond forms when atomic orbitals overlap, allowing electrons to be shared between atoms.
- The extent of orbital overlap determines the strength of the bond.
4. Sigma (σ) and Pi (π) Bonds:
- Sigma bonds result from the head-to-head overlap of orbitals. They are the first bonds formed between two atoms.
- Pi bonds result from the side-to-side overlap of p orbitals and are found in double and triple bonds, in addition to the sigma bonds.
5. Localized Electron Model:
- VBT treats electrons as localized between two atoms in a bond, unlike Molecular Orbital Theory, which treats electrons as delocalized over the entire molecule.
6. Resonance:
- Some molecules can be represented by more than one valid Lewis structure, known as resonance structures.
- VBT accounts for resonance by considering the molecule as a resonance hybrid of multiple structures.
Valence Bond Theory provides a fundamental framework for understanding chemical bonding in terms of orbital overlap and hybridization. While it has limitations, especially in explaining complex bonding scenarios, it remains a valuable tool for studying the structure and behavior of molecules.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
Комментарии
 0:10:39
0:10:39
 0:07:54
0:07:54
 0:10:33
0:10:33
 0:11:58
0:11:58
 0:07:45
0:07:45
 0:56:46
0:56:46
 0:26:04
0:26:04
 1:01:29
1:01:29
 0:25:11
0:25:11
 0:12:05
0:12:05
 0:12:40
0:12:40
 0:00:30
0:00:30
 0:59:48
0:59:48
 0:06:31
0:06:31
 0:28:44
0:28:44
 0:18:53
0:18:53
 0:24:35
0:24:35
 0:11:59
0:11:59
 0:51:25
0:51:25
 0:23:53
0:23:53
 0:13:46
0:13:46
 1:09:44
1:09:44
 0:10:55
0:10:55
 0:09:58
0:09:58