filmov
tv
Resonance and Formal Charge - AP Chem Unit 2, Topic 6

Показать описание
In this video, Mr. Krug shows how some molecules have multiple resonance structures. He also shows students how to calculate the formal charge of each atom in a molecule and how to use formal charge to determine which Lewis diagram will represent the most stable structure for a molecule.
00:00 Resonance Structures
05:01 Formal Charge
00:00 Resonance Structures
05:01 Formal Charge
Resonance Structures/Assigning Formal Charge
Resonance Structures, Basic Introduction - How To Draw The Resonance Hybrid, Chemistry
Resonance and Formal Charge - AP Chem Unit 2, Topic 6
Resonance Structures
Formal Charge
Resonance and Formal Charge
How To Calculate The Formal Charge of an Atom - Chemistry
2.6 Resonance and Formal Charge
11. Formal Charge and Resonance
Resonance and Formal Charge
Drawing Lewis Structures: Resonance Structures - Chemistry Tutorial
Resonance Made Easy! Finding the Most Stable Resonance Structure - Organic Chemistry
2.6 - Resonance and Formal Charge
Resonance and Formal Charges: Nitrate Ion
Resonance and Formal Charge
Resonance and Formal Charge.wmv
Lewis Structures II - resonance and formal charges
Formal Charge and Resonance - Explained
Lewis Sturctures: Resonance and Formal Charge
3.4 - Resonance, Formal Charge, Bond Order
Lewis Structure: Carbonate ion plus dipoles, shape, angles, resonance and formal charges
Chapter 4.5 - Formal Charges and Resonance
Formal charge on carbon | Resonance and acid-base chemistry | Organic chemistry | Khan Academy
Ch 8 Formal charge and resonance AP Chem
Комментарии
 0:12:32
0:12:32
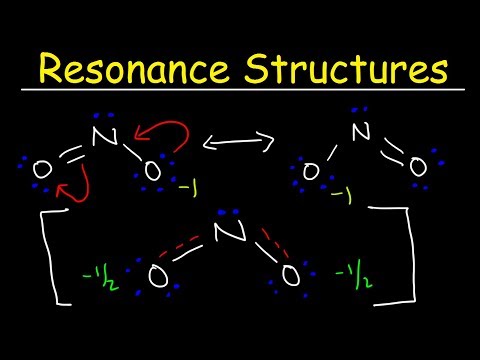 0:10:31
0:10:31
 0:12:13
0:12:13
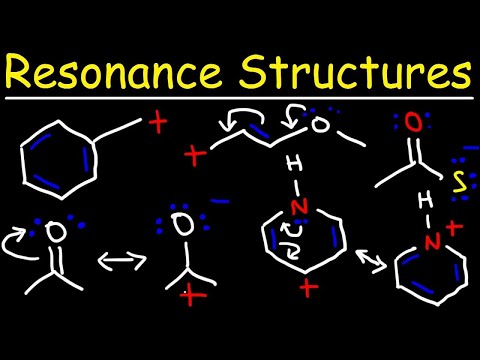 0:13:14
0:13:14
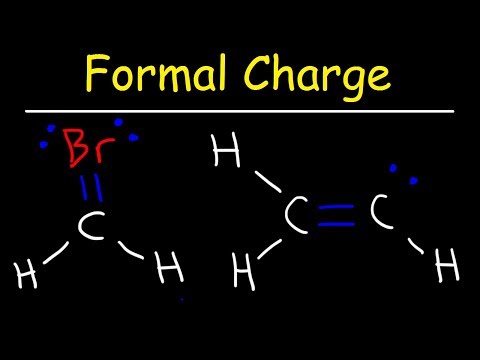 0:06:14
0:06:14
 0:09:19
0:09:19
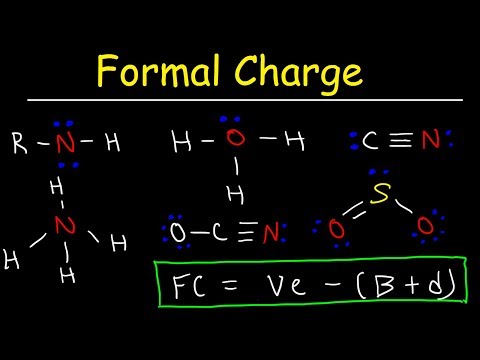 0:13:10
0:13:10
 0:16:40
0:16:40
 0:28:46
0:28:46
 0:11:46
0:11:46
 0:04:09
0:04:09
 0:08:25
0:08:25
 0:07:35
0:07:35
 0:13:29
0:13:29
 0:26:26
0:26:26
 0:08:46
0:08:46
 0:14:56
0:14:56
 0:23:17
0:23:17
 0:21:39
0:21:39
 0:07:31
0:07:31
 0:07:30
0:07:30
 0:10:08
0:10:08
 0:09:36
0:09:36
 0:14:18
0:14:18