filmov
tv
Structure of the Atom in One Shot | CBSE Class 9 Science Chapter 4 NCERT Solutions | Vedantu

Показать описание
Structure of the Atom in One Shot | CBSE Class 9 Science Chapter 4 NCERT Solutions | Vedantu Class 9 and 10. In today's session, Chemistry Master Teacher Anubha Ma’am takes you through the Chapter The Structure of an atom, as it is one of the most important topics in the NCERT Science Book, CBSE 2021 Board Exam. Class 9 Structure of Atom Problems will be discussed and solved by Anubha Ma’am in a simple and effortless way. Learn Topics Like Atomic Model, Thomson's Model.
✅ YouTube Channel Links -
✔️Learn from the Masters - India’s best teachers
✔️Access to quality education anytime, anywhere
Teach Online | At Home Learning | Classes Online | Vedantu Online Classes | Online Coaching | online course
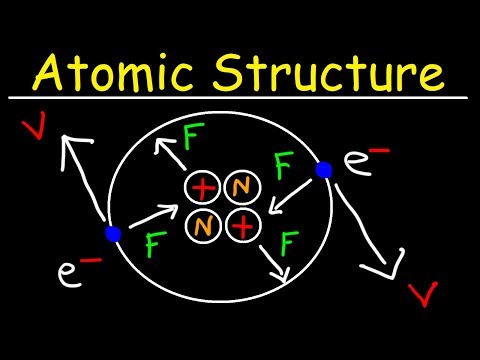
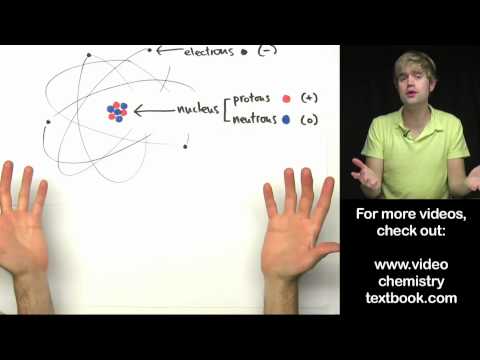
The Chapter Structure Of The Atom contains topics such as Thomson's Atomic Model, Rutherford's Model Of Atom, Alpha Particle Scattering Experiment, Bohr's Model Of Atom, Atomic Mass, Mass Number, Atomic Number, Valence Electron, Valency, Initial 20 Elements, Electronic Configuration, Concept Of Electronic Configuration, Drawback Of Rutherford's Model, Isotopes And Isobars, Formula For Maximum Electron Holding Capacity Of Orbit Or Shell
#StructureOfTheAtom #CBSEClass9 #Vedantu9and10 #StayHomeAndLearn
--------------------
Vedantu is India's leading Online tutoring company which enables students to learn LIVE with some of India's best-curated teachers. Vedantu`s USP is its quality of teachers and the way of teaching. Vedantu is not like your regular coaching for 9th and 10th class Exam Preparations.
#vedantu #learnliveonline
--------------------
Free Live Interactive with India's best Teachers on Vedantu Master Classes
Structure Of The Atom Class 9 NCERT Solutions | Structure Of The Atom Full Explanation | Structure Of The Atom Class 9 | Structure Of The Atom in English | Structure Of The Atom Class 9 CBSE | Structure Of The Atom Question and Answer | Structure Of The Atom Solution | Structure Of The Atom Valency | Structure Of The Atom NCERT | Structure Of The Atom Vedantu | Structure Of The Atom | Structure of Atom Class 9 | Class 9 Structure of Atom | Structure of Atom Explanation | Structure of Atom Valency | Structure of Atom NCERT | Structure of Atom CBSE | Structure of Atom in English | Vedantu Class 9 and 10 | Vedantu Class 10 Chemistry
✅ YouTube Channel Links -
✔️Learn from the Masters - India’s best teachers
✔️Access to quality education anytime, anywhere
Teach Online | At Home Learning | Classes Online | Vedantu Online Classes | Online Coaching | online course
The Chapter Structure Of The Atom contains topics such as Thomson's Atomic Model, Rutherford's Model Of Atom, Alpha Particle Scattering Experiment, Bohr's Model Of Atom, Atomic Mass, Mass Number, Atomic Number, Valence Electron, Valency, Initial 20 Elements, Electronic Configuration, Concept Of Electronic Configuration, Drawback Of Rutherford's Model, Isotopes And Isobars, Formula For Maximum Electron Holding Capacity Of Orbit Or Shell
#StructureOfTheAtom #CBSEClass9 #Vedantu9and10 #StayHomeAndLearn
--------------------
Vedantu is India's leading Online tutoring company which enables students to learn LIVE with some of India's best-curated teachers. Vedantu`s USP is its quality of teachers and the way of teaching. Vedantu is not like your regular coaching for 9th and 10th class Exam Preparations.
#vedantu #learnliveonline
--------------------
Free Live Interactive with India's best Teachers on Vedantu Master Classes
Structure Of The Atom Class 9 NCERT Solutions | Structure Of The Atom Full Explanation | Structure Of The Atom Class 9 | Structure Of The Atom in English | Structure Of The Atom Class 9 CBSE | Structure Of The Atom Question and Answer | Structure Of The Atom Solution | Structure Of The Atom Valency | Structure Of The Atom NCERT | Structure Of The Atom Vedantu | Structure Of The Atom | Structure of Atom Class 9 | Class 9 Structure of Atom | Structure of Atom Explanation | Structure of Atom Valency | Structure of Atom NCERT | Structure of Atom CBSE | Structure of Atom in English | Vedantu Class 9 and 10 | Vedantu Class 10 Chemistry
Комментарии
 0:02:20
0:02:20
 0:11:45
0:11:45
 0:02:02
0:02:02
 0:01:44
0:01:44
 0:30:31
0:30:31
 0:04:00
0:04:00
 0:07:44
0:07:44
 0:09:49
0:09:49
 0:27:50
0:27:50
 0:04:06
0:04:06
 0:04:58
0:04:58
 0:10:37
0:10:37
 0:07:17
0:07:17
 0:04:38
0:04:38
 0:10:43
0:10:43
 0:33:07
0:33:07
 0:02:28
0:02:28
 0:00:12
0:00:12
 1:29:48
1:29:48
 0:09:42
0:09:42
 0:03:26
0:03:26
 0:04:26
0:04:26
 1:50:52
1:50:52
 1:57:06
1:57:06