filmov
tv
Exothermic and Endothermic vs Exergonic and Endergonic (simplified)

Показать описание
One of the most common things I come across when working with students studying for the MCAT, PCAT, DAT, AP, and college level chemistry courses is understanding the differences between endergonic and exergonic vs. endothermic and exothermic.
Before we get into the differences lets point out what they have in common: energy is going somewhere. In both thermic and ergonic processes energy is either going in or out. The major difference being what kind of energy is moving in and out, and the details of that energy movement.
I am going to assume that if you are asking the difference between exo(endo)thermic and exer(ender)gonic you are probably familiarized with the differences between enthalpy, entropy, and gibbs free energy. In case you need a refresher here it is:
Entropy, S
For our discussion, Entropy is a measurement of disorder.
Enthalpy, H
The changes in potential energy involved in any process of transformation, such as breaking and forming chemical bonds in a reaction.
For our discussion we can just conflate this with the flow of heat under constant pressure conditions: see below.
Gibbs free energy, G
The energy of a chemical reaction you can use to do work.
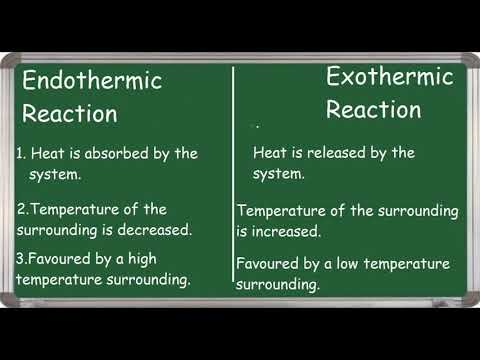
When we measure changes such as exothermic or endothermic processes: we are measuring changes in potential energy involved in the formation and breaking of chemical bonds in a particular reaction (exo and endothermic).
AN: If you are really into the detailed differences between enthalpy of reaction from enthalpy of formation, click here. For our discussion ill just identify them as the same since they have little relevance to understanding the definition of exo and endothermic.
Changes in enthalpy: either exothermic or endothermic manifest themselves as the flow of heat ( changes in kinetic energy of particles in our reaction) under constant pressure conditions, and can be measured as changes in potential energy circa the first law of thermodynamics and the definition of enthalpy. ******
So in exothermic and endothermic processes POTENTIAL ENERGY is changing as energy flows in and out.
Before we get into the differences lets point out what they have in common: energy is going somewhere. In both thermic and ergonic processes energy is either going in or out. The major difference being what kind of energy is moving in and out, and the details of that energy movement.
I am going to assume that if you are asking the difference between exo(endo)thermic and exer(ender)gonic you are probably familiarized with the differences between enthalpy, entropy, and gibbs free energy. In case you need a refresher here it is:
Entropy, S
For our discussion, Entropy is a measurement of disorder.
Enthalpy, H
The changes in potential energy involved in any process of transformation, such as breaking and forming chemical bonds in a reaction.
For our discussion we can just conflate this with the flow of heat under constant pressure conditions: see below.
Gibbs free energy, G
The energy of a chemical reaction you can use to do work.
When we measure changes such as exothermic or endothermic processes: we are measuring changes in potential energy involved in the formation and breaking of chemical bonds in a particular reaction (exo and endothermic).
AN: If you are really into the detailed differences between enthalpy of reaction from enthalpy of formation, click here. For our discussion ill just identify them as the same since they have little relevance to understanding the definition of exo and endothermic.
Changes in enthalpy: either exothermic or endothermic manifest themselves as the flow of heat ( changes in kinetic energy of particles in our reaction) under constant pressure conditions, and can be measured as changes in potential energy circa the first law of thermodynamics and the definition of enthalpy. ******
So in exothermic and endothermic processes POTENTIAL ENERGY is changing as energy flows in and out.
Комментарии
 0:03:31
0:03:31
 0:11:51
0:11:51
 0:04:17
0:04:17
 0:01:49
0:01:49
 0:02:32
0:02:32
 0:03:01
0:03:01
 0:04:26
0:04:26
 0:03:23
0:03:23
 0:10:45
0:10:45
 0:03:11
0:03:11
 0:02:41
0:02:41
 0:03:46
0:03:46
 0:07:35
0:07:35
 0:00:29
0:00:29
 0:04:07
0:04:07
 0:00:39
0:00:39
 0:19:02
0:19:02
 0:06:35
0:06:35
 0:00:30
0:00:30
 0:03:14
0:03:14
 0:02:23
0:02:23
 0:05:17
0:05:17
 0:14:19
0:14:19
 0:09:26
0:09:26