filmov
tv
What Are Endothermic & Exothermic Reactions | Chemistry | FuseSchool

Показать описание
What Are Endothermic & Exothermic Reactions | Chemistry | FuseSchool
An exothermic reaction gives off energy to the surroundings; like a fire giving off heat. An endothermic reaction takes in energy from the surroundings; like a snowman melting.
Exothermic reactions transfer energy to the surroundings, and this energy is usually heat energy, they cause the surroundings to heat up. Just like a bonfire keeping everyone warm.
As well as combustion (burning), other examples of exothermic reactions are:
- Neutralisation reactions between acids and alkalis
- The reaction between water and calcium oxide
- Respiration.
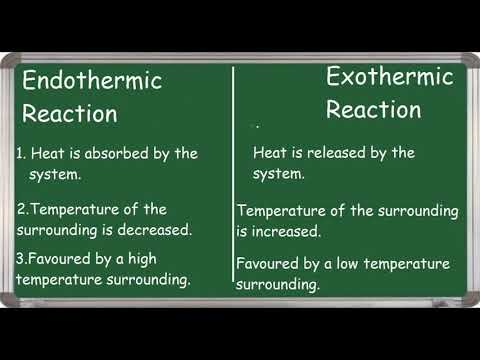
It is easy to detect an exothermic reaction - just get your thermometer and see if the temperature increases. Most chemical reactions are exothermic, because heat is given out.
Physical processes can also be endothermic or exothermic. When something freezes, it goes from liquid to solid. Bonds need to be made for this to happen, and to make bonds you need to do some work, thus energy is given out and freezing is exothermic.
Similarly, when condensation happens - because a gas is going to liquid, again bonds need to be made and so energy is given out. So freezing and condensation are exothermic. Because in exothermic reactions, energy is given out to the surroundings. This means that the energy of the reactants is higher than the energy of the products.
Endothermic reactions are less common. They take in energy from the surroundings. The energy being transferred is usually heat. So in endothermic reactions, the surroundings usually get colder.
Some examples of endothermic reactions are:
- Electrolysis
- The reaction between sodium carbonate and ethanoic acid
- Photosynthesis.
Endothermic reactions can also be seen in physical processes. When something melts it goes from a solid to a liquid. For this to happen, bonds need to be broken. And to break bonds, energy needs to be put in. Boiling is also endothermic because energy needs to be put in to break the bonds for the liquid to turn to gas. Because in endothermic reactions, energy is added to the reaction, the energy of the products is higher than the energy of the reactants. And again, we can detect endothermic reactions with a thermometer because the temperature would get colder.
SUPPORT US ON PATREON
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
An exothermic reaction gives off energy to the surroundings; like a fire giving off heat. An endothermic reaction takes in energy from the surroundings; like a snowman melting.
Exothermic reactions transfer energy to the surroundings, and this energy is usually heat energy, they cause the surroundings to heat up. Just like a bonfire keeping everyone warm.
As well as combustion (burning), other examples of exothermic reactions are:
- Neutralisation reactions between acids and alkalis
- The reaction between water and calcium oxide
- Respiration.
It is easy to detect an exothermic reaction - just get your thermometer and see if the temperature increases. Most chemical reactions are exothermic, because heat is given out.
Physical processes can also be endothermic or exothermic. When something freezes, it goes from liquid to solid. Bonds need to be made for this to happen, and to make bonds you need to do some work, thus energy is given out and freezing is exothermic.
Similarly, when condensation happens - because a gas is going to liquid, again bonds need to be made and so energy is given out. So freezing and condensation are exothermic. Because in exothermic reactions, energy is given out to the surroundings. This means that the energy of the reactants is higher than the energy of the products.
Endothermic reactions are less common. They take in energy from the surroundings. The energy being transferred is usually heat. So in endothermic reactions, the surroundings usually get colder.
Some examples of endothermic reactions are:
- Electrolysis
- The reaction between sodium carbonate and ethanoic acid
- Photosynthesis.
Endothermic reactions can also be seen in physical processes. When something melts it goes from a solid to a liquid. For this to happen, bonds need to be broken. And to break bonds, energy needs to be put in. Boiling is also endothermic because energy needs to be put in to break the bonds for the liquid to turn to gas. Because in endothermic reactions, energy is added to the reaction, the energy of the products is higher than the energy of the reactants. And again, we can detect endothermic reactions with a thermometer because the temperature would get colder.
SUPPORT US ON PATREON
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
These videos can be used in a flipped classroom model or as a revision aid.
Комментарии
 0:04:17
0:04:17
 0:05:21
0:05:21
 0:01:49
0:01:49
 0:10:45
0:10:45
 0:01:15
0:01:15
 0:03:23
0:03:23
 0:06:25
0:06:25
 0:04:35
0:04:35
 0:23:07
0:23:07
 0:05:43
0:05:43
 0:05:59
0:05:59
 0:07:13
0:07:13
 0:02:41
0:02:41
 0:06:35
0:06:35
 0:01:40
0:01:40
 0:03:11
0:03:11
 0:02:23
0:02:23
 0:00:24
0:00:24
 0:08:09
0:08:09
 0:02:07
0:02:07
 0:02:28
0:02:28
 0:10:52
0:10:52
 0:03:01
0:03:01
 0:08:46
0:08:46