filmov
tv
Exergonic vs endergonic reaction diagrams

Показать описание
ERROR: I keep saying deltaG when I just mean G (Gibbs free energy). The delta is referring to the change. The axis is just G and deltaG is the difference between the reactants and the products.
Exergonic vs endergonic reaction diagrams
endergonic and exergonic reactions
Endergonic, exergonic, exothermic, and endothermic reactions | Khan Academy
Ender and exergonic reactions
Endergonic and Exergonic Reactions
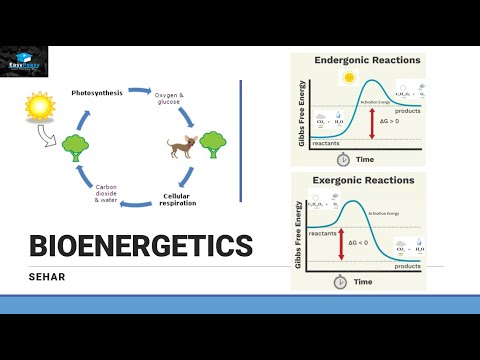
Introduction of Bioenergetics Part 1
Exothermic and Endothermic vs Exergonic and Endergonic (simplified)
Endergonic and Exergonic Reactions in the Cell | Cell Bio | Video Textbooks - Preview
Endothermic Vs. Exothermic Reaction Graphs
GCSE Chemistry - Exothermic and Endothermic Reactions #43
What Are Endothermic & Exothermic Reactions | Chemistry | FuseSchool
endergonic reactions
Endothermic and Exothermic Reactions
Enzyme/ Energy Reaction Graphs
Chapter 8 Endergonic and Exergonic Reactions
Exothermic vs Endothermic Chemical Reactions
Energy, Work, Endergonic vs Exergonic
Understanding Exergonic & Endergonic Reactions
Cell Biology - Exergonic and Endergonic reactions
Identifying an Endothermic and Exothermic Reaction Trick #Shorts
Exergonic vs Endergonic Reactions
Coupled Reactions | Cell Bio | Video Textbooks - Preview
Exergonic Vs. Endergonic reactions
Energy_ChemicalReactions (cc only)
Комментарии
 0:04:26
0:04:26
 0:02:32
0:02:32
 0:11:51
0:11:51
 0:07:35
0:07:35
 0:05:17
0:05:17
 0:12:40
0:12:40
 0:03:31
0:03:31
 0:00:23
0:00:23
 0:03:01
0:03:01
 0:05:21
0:05:21
 0:04:17
0:04:17
 0:06:00
0:06:00
 0:04:35
0:04:35
 0:03:54
0:03:54
 0:13:59
0:13:59
 0:01:49
0:01:49
 0:19:02
0:19:02
 0:00:39
0:00:39
 0:03:25
0:03:25
 0:00:13
0:00:13
 0:06:54
0:06:54
 0:00:23
0:00:23
 0:00:56
0:00:56
 0:06:10
0:06:10