filmov
tv
Ionic Radius Trends - Periodic Table - Chemistry Class 11

Показать описание
Ionic Radius Trends Video Lecture from Periodic table Chapter of Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.
Watch Previous Videos of Chapter Periodic table:-
Watch Next Videos of Chapter Periodic table:-
#ChemistryClass11
#ChemistryClass11JEE
#ChemistryClass11Lectures
#ChemistryClass11Tutorial
#OnlineVideoLectures
#EkeedaOnlineLectures
#EkeedaVideoLectures
#EkeedaVideoTutorial
Thanks For Watching. You can follow and Like us on following social media.
Happy Learning : )
Watch Previous Videos of Chapter Periodic table:-
Watch Next Videos of Chapter Periodic table:-
#ChemistryClass11
#ChemistryClass11JEE
#ChemistryClass11Lectures
#ChemistryClass11Tutorial
#OnlineVideoLectures
#EkeedaOnlineLectures
#EkeedaVideoLectures
#EkeedaVideoTutorial
Thanks For Watching. You can follow and Like us on following social media.
Happy Learning : )
Ionic Radius Trends, Basic Introduction, Periodic Table, Sizes of Isoelectric Ions, Chemistry
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Ionic Radius | Trends of Ionic Radius in Periodic Table
Ionic Radius Trend of the Periodic Table | Metal and Nonmetal Ionic Radii Trend
Periodic Trends: Ionic Radius (Ionic Size) | Study Chemistry With Us
Periodic Trends: Atomic Radius
46: Periodic trends: Ionic radius
Practice Problem: Atomic Radii and Ionic Radii
Periodic Trends - Atomic Radius, Electronegativity, Ionization Energy - Chemistry Series
Ionic Radius Trends - Periodic Table - Chemistry Class 11
Periodic Trends Practice Problems: Ionic Radius (Ionic Size) | Study Chemistry With Us
Atomic and ionic radii | Periodic table | Chemistry | Khan Academy
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE
What is Atomic Radius? Periodic Trends
Trends in the Periodic Table
Atomic radius trends on periodic table | Periodic table | Chemistry | Khan Academy
IONIC RADIUS#IONIC RADII TRENDS ACROSS THE PERIODIC TABLE
Periodic Trends: Atomic Radius | Study Chemistry With Us
Atomic Radius - Basic Introduction - Periodic Table Trends, Chemistry
periodic trends pt1: Atomic and Ionic Radius
Periodic trends and Coulomb's law | Atomic structure and properties | AP Chemistry | Khan Acade...
Atomic and Ionic Radius Trends in the Periodic Table
Atomic Radius Periodic Trends made EASY 🤗👍 #chemistry #science #education #shorts #short
Ionic Radius | Periodic Table class 11 | IIT JEE/NEET | Poonam mam | ATP STAR KOTA
Комментарии
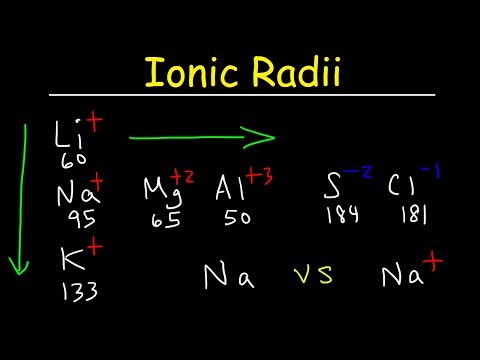 0:11:47
0:11:47
 0:07:53
0:07:53
 0:09:00
0:09:00
 0:06:32
0:06:32
 0:16:10
0:16:10
 0:03:47
0:03:47
 0:07:38
0:07:38
 0:07:27
0:07:27
 0:18:06
0:18:06
 0:05:17
0:05:17
 0:11:18
0:11:18
 0:11:26
0:11:26
 0:24:55
0:24:55
 0:08:04
0:08:04
 0:09:49
0:09:49
 0:09:40
0:09:40
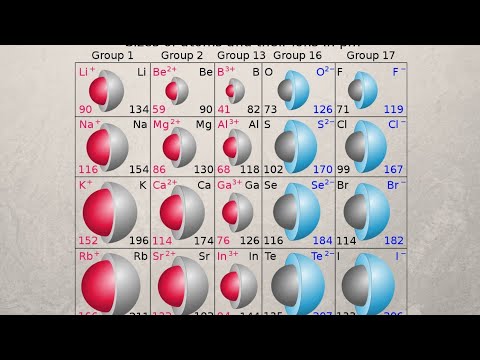 0:07:33
0:07:33
 0:17:39
0:17:39
 0:14:04
0:14:04
 0:10:50
0:10:50
 0:10:43
0:10:43
 0:04:13
0:04:13
 0:00:54
0:00:54
 0:23:58
0:23:58