filmov
tv
Phase Change to Supercritical CO2

Показать описание
In this short video, liquid CO2 is pumped into a windowed pressure vessel and while the pressure increases, the viewer watches the CO2 change phases past the CO2 critical point into the supercritical region. Supercritical CO2 is no longer distinguishable between a liquid or a gas. The CO2 pressure vessel is then depressurized, and the viewer can see the phases separate as the pressure is let down.
Phase Change to Supercritical CO2
What Happens When a Liquid Turns Supercritical?
This is not a liquid or a gas
A close look at supercritical carbon dioxide CO2
Supercritical carbon dioxide (sCO2) | How does it look like?
Thermodynamics - Explaining the Critical Point
supercritical fluids
Phase Diagrams: Triple Points, Critical Points and Supercritical Fluids
Supercritical fluids, a state between Liquid and Gas
Supercritical CO2! #science #physics #supercritical
Can a Boat Float In Supercritical Fluid?
How Can Matter Be BOTH Liquid AND Gas?
Carbon Dioxide Phase Transformations
Going supercritical.
Carbon Dioxide - Phase Transitions
Supercritical CO2 Sterilization Initial Fill in a NovaSterilis Nova2200
how supercritical fluids give you decaf coffee
EffecTech supercritical CO2 demonstration
Triple Point of Water
Supercritical CO2 Turbines Explained {Future Friday Ep92}
11.2 Phase Diagrams | General Chemistry
CO2 Phase Changes | Danfoss Climate Solutions
Properties of superheated steam
Chem 117 Week 4 Lecture 4 Supercritical Carbon Dioxide
Комментарии
 0:01:45
0:01:45
 0:05:55
0:05:55
 0:01:00
0:01:00
 0:07:59
0:07:59
 0:02:00
0:02:00
 0:04:33
0:04:33
 0:04:06
0:04:06
 0:04:51
0:04:51
 0:12:14
0:12:14
 0:00:16
0:00:16
 0:09:13
0:09:13
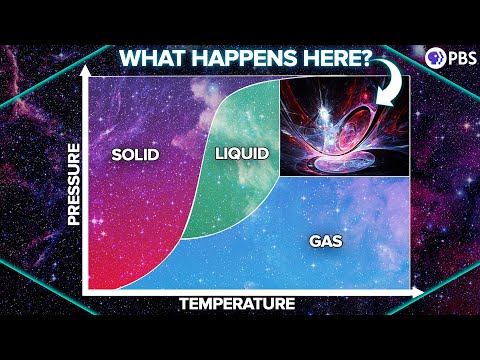 0:21:17
0:21:17
 0:02:11
0:02:11
 0:19:53
0:19:53
 0:01:06
0:01:06
 0:00:15
0:00:15
 0:00:56
0:00:56
 0:02:20
0:02:20
 0:01:55
0:01:55
 0:16:30
0:16:30
 0:14:45
0:14:45
 0:09:07
0:09:07
 0:04:35
0:04:35
 0:12:52
0:12:52