filmov
tv
supercritical fluids

Показать описание
liquid CO2 is heated in a pressure cell until it reaches the critical point were it changes into a supercritical fluid
What Happens When a Liquid Turns Supercritical?
Supercritical fluids, a state between Liquid and Gas
Can a Boat Float In Supercritical Fluid?
This is not a liquid or a gas
Supercritical Fluids Explained
supercritical fluids
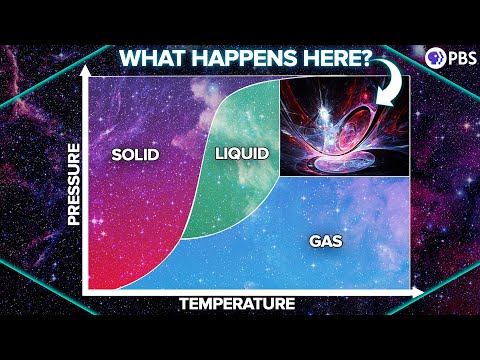
Phase Diagrams: Triple Points, Critical Points and Supercritical Fluids
Going supercritical.
Supercritical carbon dioxide (sCO2) | How does it look like?
Can a Boat Float In Supercritical Fluid? (Stabilized Version)
How Can Matter Be BOTH Liquid AND Gas?
Supercritical Fluids
how supercritical fluids give you decaf coffee
How To Do Supercritical CO2 Extraction
Thermodynamics - Explaining the Critical Point
Supercritical Fluids Extraction Process Explained
A close look at supercritical carbon dioxide CO2
Supercritical Fluids
Recycling using supercritical fluids | CNRS in English
Supercritical Planets Discovery - Gas and Liquid At the Same Time
The Unknown States of Matter- Supercritical Fluids
Element 61 - Sub- and Super-Critical Fluids
Neither Gas nor Liquid - Supercritical CO2 #science #chemistry
Liquid water vs Supercritical fluid water
Комментарии
 0:05:55
0:05:55
 0:12:14
0:12:14
 0:09:13
0:09:13
 0:01:00
0:01:00
 0:02:54
0:02:54
 0:04:06
0:04:06
 0:04:51
0:04:51
 0:19:53
0:19:53
 0:02:00
0:02:00
 0:09:13
0:09:13
 0:21:17
0:21:17
 0:02:58
0:02:58
 0:00:56
0:00:56
 0:01:57
0:01:57
 0:04:33
0:04:33
 0:01:45
0:01:45
 0:07:59
0:07:59
 0:03:27
0:03:27
 0:07:05
0:07:05
 0:12:06
0:12:06
 0:07:24
0:07:24
 0:02:08
0:02:08
 0:01:00
0:01:00
 0:01:07
0:01:07