filmov
tv
Solving a Rate Law Using the Initial Rates Method

Показать описание
Solving a Rate Law Using the Initial Rates Method
How to Find the Rate Law and Rate Constant (k)
Kinetics: Initial Rates and Integrated Rate Laws
Chemical Kinetics - Initial Rates Method
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
Worked example: Determining a rate law using initial rates data | AP Chemistry | Khan Academy
Working out order from a rate table - tricky example
14.2 Rate Laws | General Chemistry
APSC MAINS II SOLVE CASE STUDY WITH SINGHAM II SESSION 1 II 1st TIME ANYWHERE II APSC COACHING
ALEKS: Using a rate law
Reaction Order Tricks & How to Quickly Find the Rate Law
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics
Rate Law Problems
Determining Rate Law
Kinetics: Using the Integrated Rate Laws and Graphs to Determine the Rate Law
DON'T MISS THIS Rate Law and Rate Constant Question
Rate Law
Steady-State Approximation, Rate Law, Kinetics - Chemistry
Rate law calculations when one reactant is not held constant
S12E2 - Rate Laws: Differential Rate Laws and the Integrated Rate Law
Reaction mechanism and rate law | Kinetics | AP Chemistry | Khan Academy
Removing Intermediates from Rate Laws
Determine Rate Law from Reaction Mechanisms, Fast then Slow Step: Part I
Determine Rate Law from Reaction Mechanism, Fast then Slow Step: Part 2
Комментарии
 0:10:49
0:10:49
 0:03:42
0:03:42
 0:09:10
0:09:10
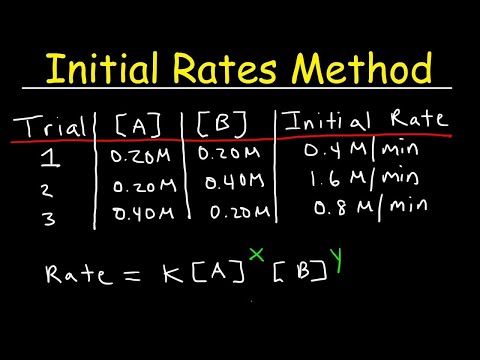 0:34:53
0:34:53
 0:18:48
0:18:48
 0:12:28
0:12:28
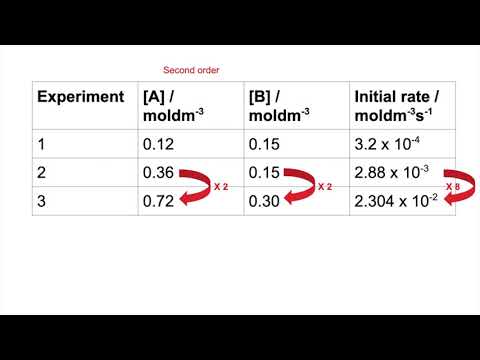 0:02:46
0:02:46
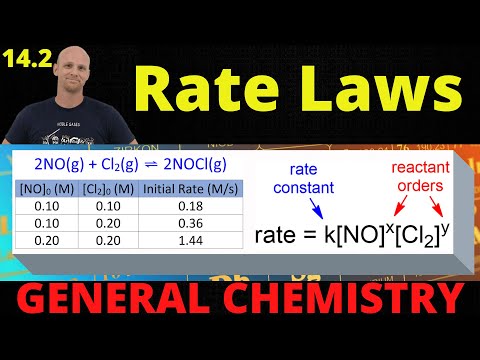 0:25:16
0:25:16
 0:16:33
0:16:33
 0:04:04
0:04:04
 0:01:58
0:01:58
 0:48:46
0:48:46
 0:18:54
0:18:54
 0:12:35
0:12:35
 0:09:55
0:09:55
 0:03:46
0:03:46
 0:13:35
0:13:35
 0:03:14
0:03:14
 0:12:43
0:12:43
 0:12:49
0:12:49
 0:08:42
0:08:42
 0:09:28
0:09:28
 0:07:46
0:07:46
 0:06:37
0:06:37